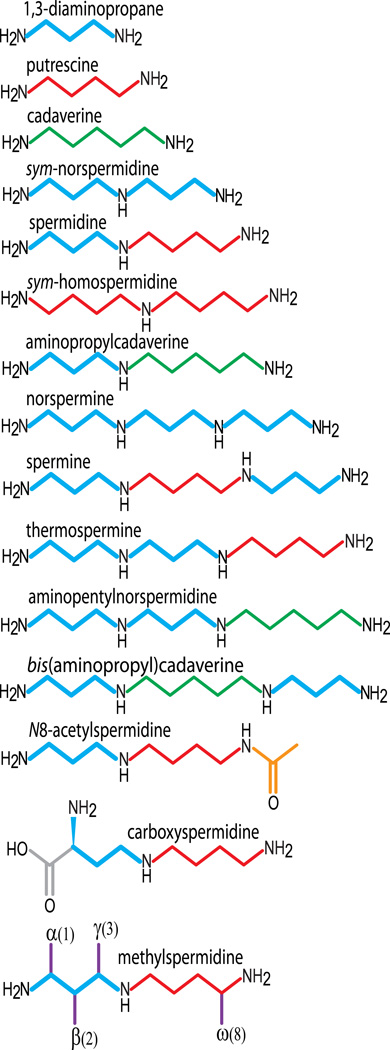
Figure 2.
Natural and synthetic polyamines used in this study. The 1,3-diaminopropane moiety in each molecule is shown in bold blue, putrescine (1,4-diaminobutane) in red and cadaverine in green (1,5-diaminopentane). Carboxyspermidine accumulates only in the ΔCASDC mutant. Each of the methylated carbon positions (in purple) of the methylated spermidine analogues is displayed on the same molecule.