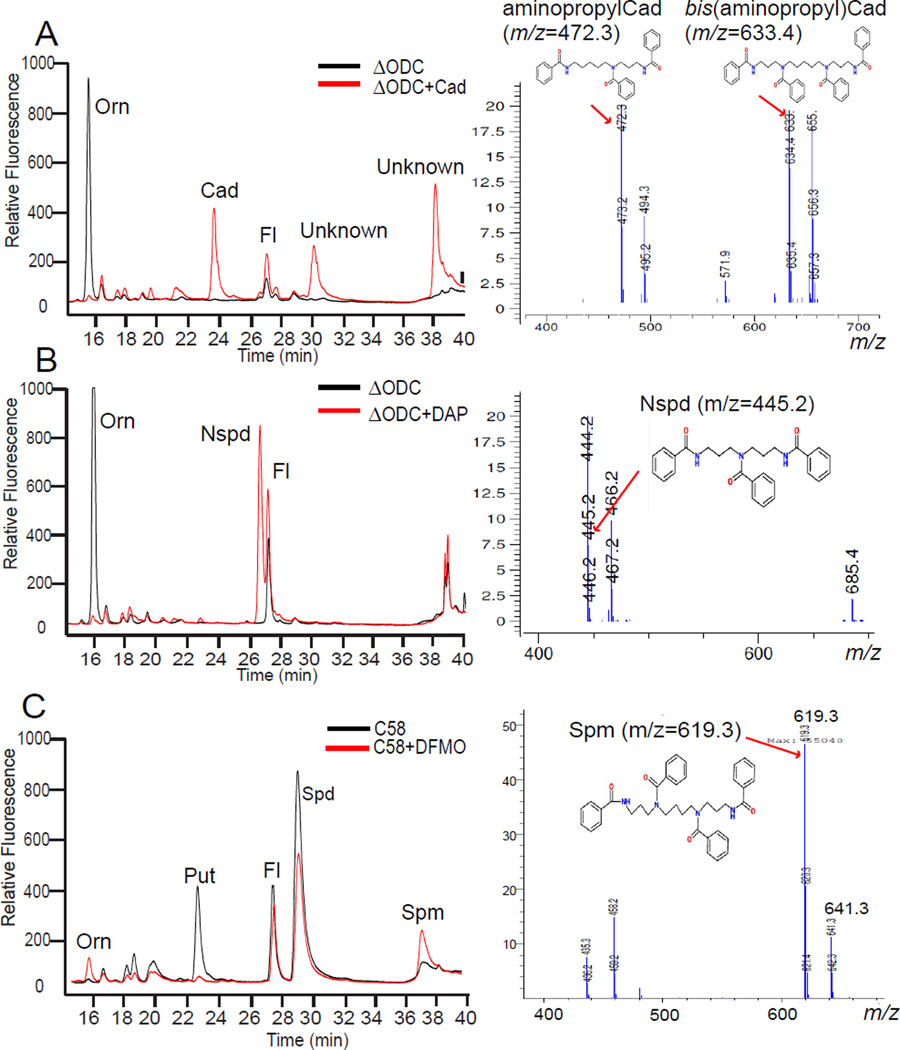
Figure 4.
Polyamine biosynthetic plasticity in A. tumefaciens C58. The left panels are HPLC analyses and on the right are independent LC-MS mass spectra. (A) The ΔODC strain grown with or without 100 µM cadaverine (Cad). The unknown peak at 38.5 min in the HPLC trace is either the tetraamine bis(aminopropyl)cadaverine or its isomer N1-aminopentylnorspermidine, which are not distinguished by the LC-MS analysis. (B) The ΔODC strain grown with or without 100 µM 1,3-diaminopropane (DAP). The tetraamine norspermine is not formed. (C) The parental A. tumefaciens C58 wild-type strain grown with 10 mM DFMO (α-difluoromethylornithine), a highly specific suicide inhibitor of ornithine decarboxylase (ODC). The accumulation of tetraamine spermine (Spm) or its isomer thermospermine is confirmed by LC-MS. Sodium adducted forms of the polyamines are present as ions with a 22 Da higher m/z. Orn, ornithine; Fl, AccQ-Fluor tag.