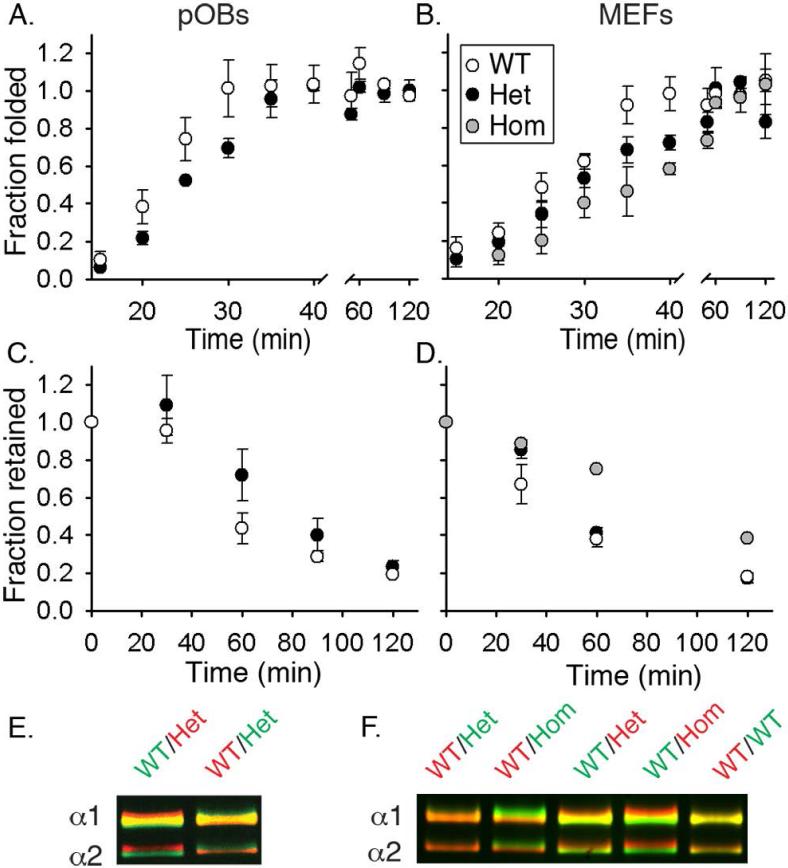
Fig. 2.
Folding, retention and post-translational overmodification of procollagen in cultured pOBs (A,C,E) and MEFs (B,D,F). (A,B) Formation of folded (chymotrypsin/ trypsin resistant), Aha-labeled molecules after a 10 min labeling pulse. 50% folding was observed in ~21 min (WT pOBs), ~ 25 min (Het pOBs), ~ 25 min (WT MEFs), ~ 30 min (Het MEFs), and ~ 35 min (Hom MEFs). (C,D) Fraction of Aha-labeled procollagen remaining inside the cell during a 2 h chase in a Met medium preceded by a 2 h Aha-labeling pulse. 50% retention was observed in ~ 50 min (WT pOBs), ~ 80 min (Het pOBs), ~ 45 min (WT MEFs), ~65 min (Het MEFs), and ~ 100 min (Hom MEFs). (E,F) Procollagen overmodification visualized by delayed electrophoretic migration of the α1(I) and α2(I) chains from triple helical domains purified by pepsin treatment and salt fractionation. Each gel lane contains a binary mixture of molecules from WT cells labeled with either AlexaFluor 488 (green) or Cy5 (red) and molecules from WT, Het or Hom cells labeled with the other dye. Vertical color separation reveals subtle differences in electrophoretic migration of the chains (38). All error bars in this and subsequent figures show the standard error of the mean measured in at least triplicate experiments.