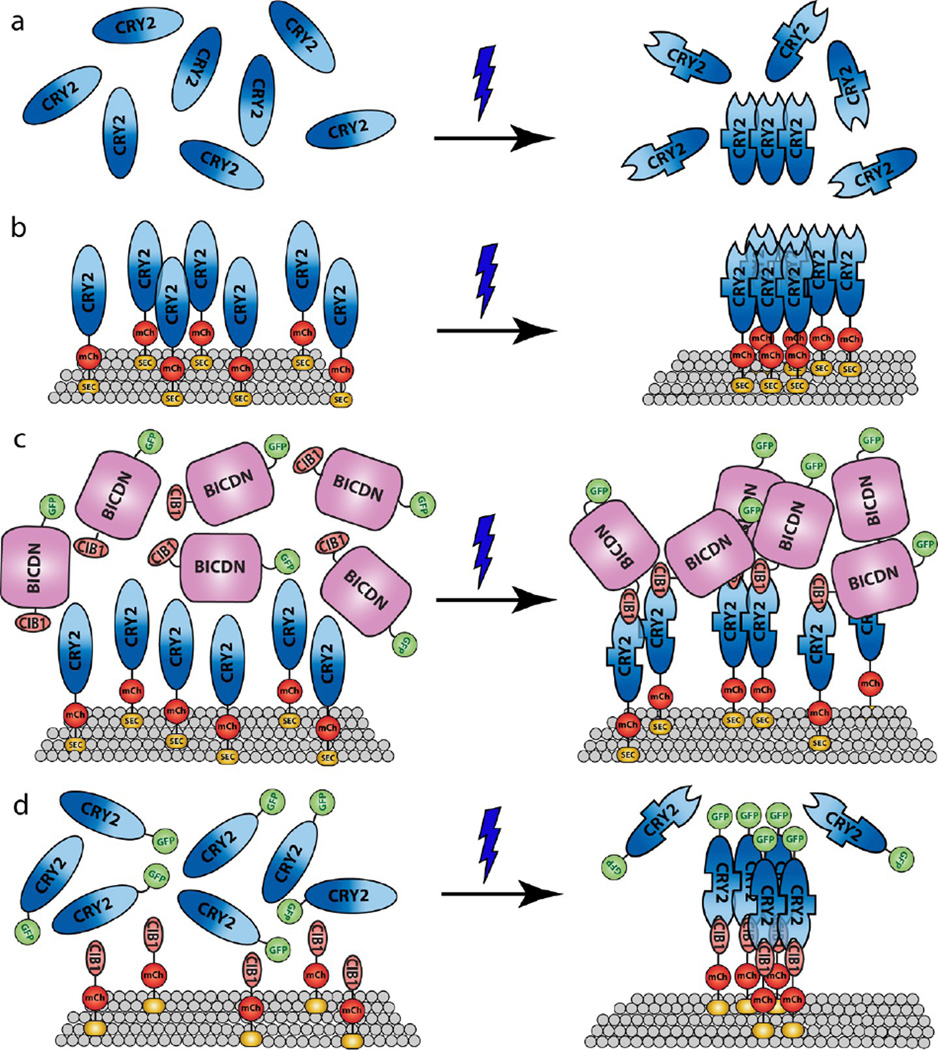
Figure 7.
Proposed mechanism of CRY2-CRY2 oligomerization and CRY2-CIB1 heterodimerization. (a) Blue light induces conformational changes in CRY2, with CRY2-CRY2 binding and CRY2-CIB1 binding occurring at different CRY2 sites. Cytoplasmic CRY2 has low oligomerization activity due to random (unaligned) protein orientation. (b) CRY2 bound on the lipid membrane has enhanced oligomeric activity likely due to preferred parallel orientation for CRY2-CRY2 binding. (c) CIB1 linked with a bulky protein domain such as BICDN can suppress CRY2 oligomerization due to steric blocking of the CRY2-CRY2 binding site. (d) Cytoplasmic CRY2 can be recruited to the cell membrane by binding to membrane-linked CIB1, which significantly enhances CRY2 oligomerization.