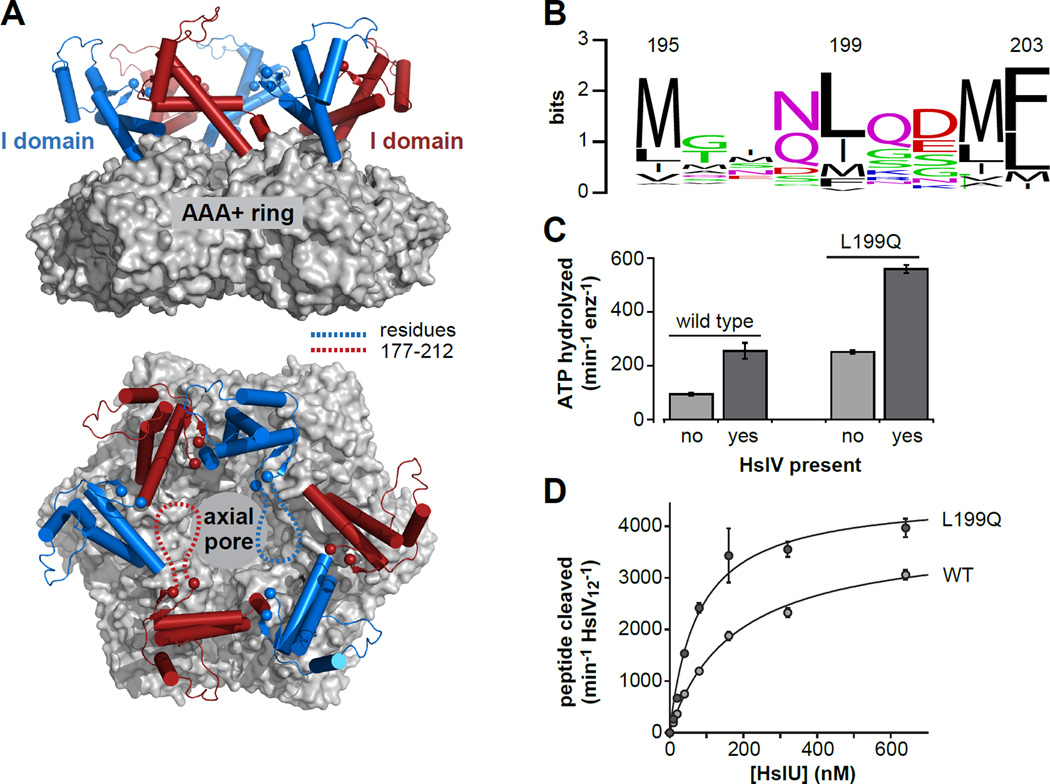
Figure 1. The HslU hexamer and initial characterization of L199QHslU.
(A) Views of a wild-type HslU hexamer (pdb 1do0; Bochtler et al., 2000) from the side and from above the axial pore. The hexameric AAA+ ring is shown in surface representation and colored light grey. The I domains are shown in cartoon representation and colored blue or red. The α-carbons of residues 176 and 213 are shown as spheres. The intervening residues (177–212) are not observed in this or other HslU structures, and are schematically depicted as dashed lines in two I domains in the bottom panel. (B) WebLogo representation (Crooks et al., 2004) of sequence conservation for HslU residue 199 and flanking amino acids. (C) The rate of hydrolysis of 5 mM ATP at 37 °C was assayed for L199QHslU and WTHslU (300 nM each) in the absence or presence of HslV (900 nM) using a coupled assay (Nørby, 1988). (D) Increasing concentrations of L199QHslU or WTHslU were titrated against HslV12 (5 nM) in the presence of 5 mM ATP at 25 °C and the rate of cleavage of a Z-GGL-AMC tripeptide (200 nM) was monitored by increased fluorescence. The lines are fits to a hyperbolic binding equation. For L199QHslUV, half-maximal activation was observed at a HslU hexamer concentration of 78 ± 14 nM and maximal activity was 4580 ± 262 peptides cleaved min−1 HslV12−1. For WTHslUV, these values were 176 ± 16 nM and 3820 ± 137 peptides cleaved min−1 HslV12−1, respectively. In panels C and D, values are averages of 3 independent replicates ± 1 SD.