Figure 1.
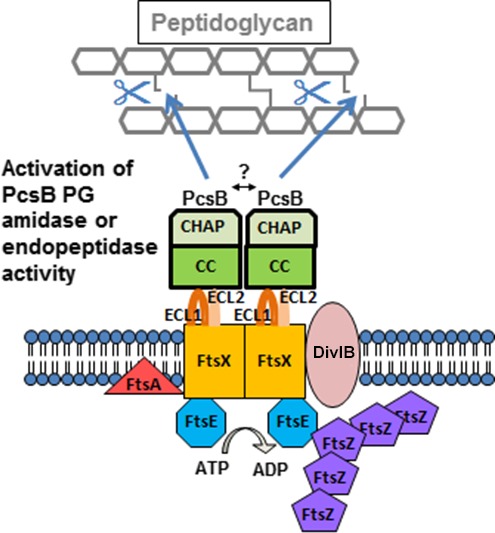
Model for regulated PG hydrolysis by the FtsEX:PcsB complex, whose function is essential in S. pneumoniae. The figure is modified from (Sham et al. 2011). The FtsEX:PcsB complex consists of the FtsE ATPase, the FtsX transmembrane protein, and the autoinhibited PcsB PG hydrolase. The FtsEX complex shares structural similarities to an ABC transporter, but does not transport a ligand. FtsX contains the large extracellular loop domain ECL1 and a small extracellular loop domain ECL2. Both extracellular loops are important in FtsEX:PcsB signal transduction. ECL1 interacts with the coiled‐coil (CC) domain of the PcsB PG hydrolase, which contains an autoinhibited CHAP catalytic domain. When activated, PcsB acts as a PG remodeling amidase or endopeptidase that cleaves PG peptides. At a specific point in cell division, the ATPase activity of FtsE is stimulated by interactions with midcell division proteins, such as FtsZ. The FtsE ATPase activity drives conformational changes in the transmembrane domain of FtsX, which also may interact with divisome proteins. The conformational change in the FtsX transmembrane domain is transmitted to the FtsX extracellular loops, ECL1 and ECL2, which in turn relieve the autoinhibition of the PcsB CHAP domain, allowing timed PG cleavage. The model is based on references (Arends et al. 2009; Sham et al. 2011, 2013; Mavrici et al. 2014; Yang et al. 2011, 2012; Meisner et al. 2013; Bartual et al. 2014; Mir et al. 2015). See text for additional details.
