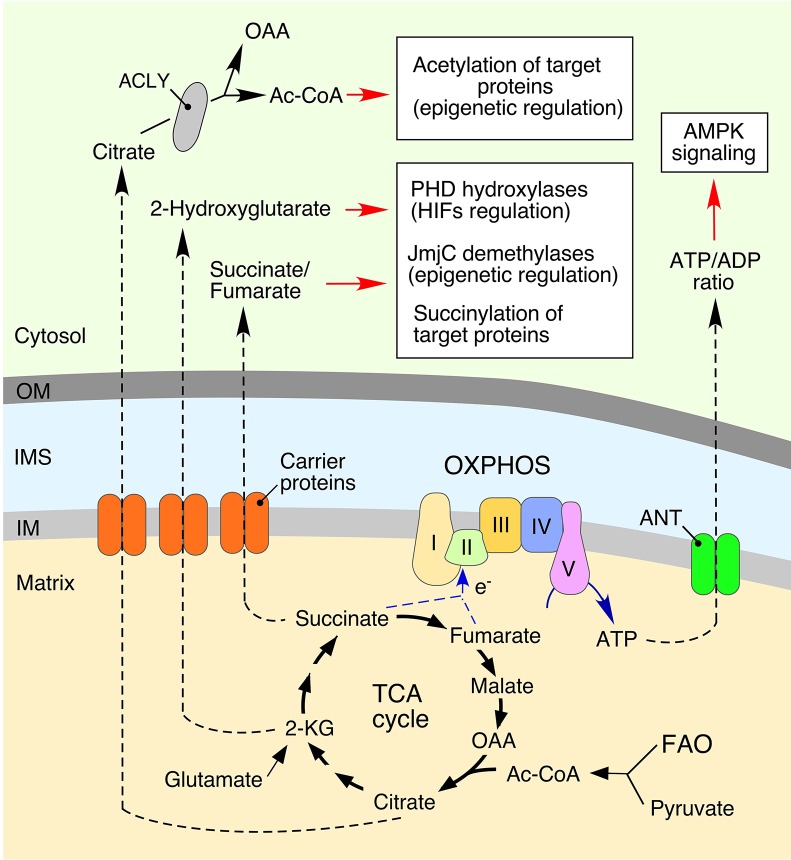
Figure 1.
Mitochondria-derived metabolites regulate various cellular processes. Bioenergetic function of mitochondria is associated with generation of ATP (adenosine triphosphate) via the oxidative phosphorylation (OXPHOS) system. This process involves conversion of various substrates imported from the cytosol via the tricarboxylic acid (TCA) cycle or fatty acid oxidation (FAO). Metabolic intermediates can be exported into the cytosol to impact various signaling cascades. Mitochondria-derived citrate is converted to oxaloacetate (OAA) and acetyl coenzyme A (Ac-CoA) by ATP citrate lyase (ACLY). Subsequently, a fraction of the cytosolic Ac-CoA can be used in protein acetylation, thereby impacting multiple cellular processes (Choudhary et al., 2009; Spange et al., 2009; Wellen et al., 2009; Eisenberg et al., 2014). Another TCA cycle biosynthetic intermediate, 2-ketoglutarate (α-KG), can be converted to 2-Hydroxyglutarate. This metabolite inhibits: (1) Jumonji C domain-containing demethylases (JmjC), affecting epigenetic reprogramming (Pearce et al., 2013; Zong et al., 2016); and (2) hypoxia-inducible prolyl hydroxylases (PHDs), thus stabilizing the hypoxia-inducible factors (HIFs) and inducing a hypoxic response (Xu et al., 2011; Pearce et al., 2013; Zong et al., 2016). The TCA metabolite, fumarate, can also act as a signal-modulating factor via its ability to bind lysine residues of various proteins, resulting in a post-translational modification called succinylation. The adenine nucleotide translocator (ANT) mediates an exchange of ATP and cytosol-derived ADP (adenosine diphosphate) across the inner mitochondrial membrane (IM). Mitochondrial dysfunction derived ATP depletion perturbs ATP/ADP ratios by subsequent activation of adenosine monophosphate-activated protein kinase (AMPK), a master regulator of cellular energy homeostasis (Hall et al., 2013; Pearce et al., 2013).