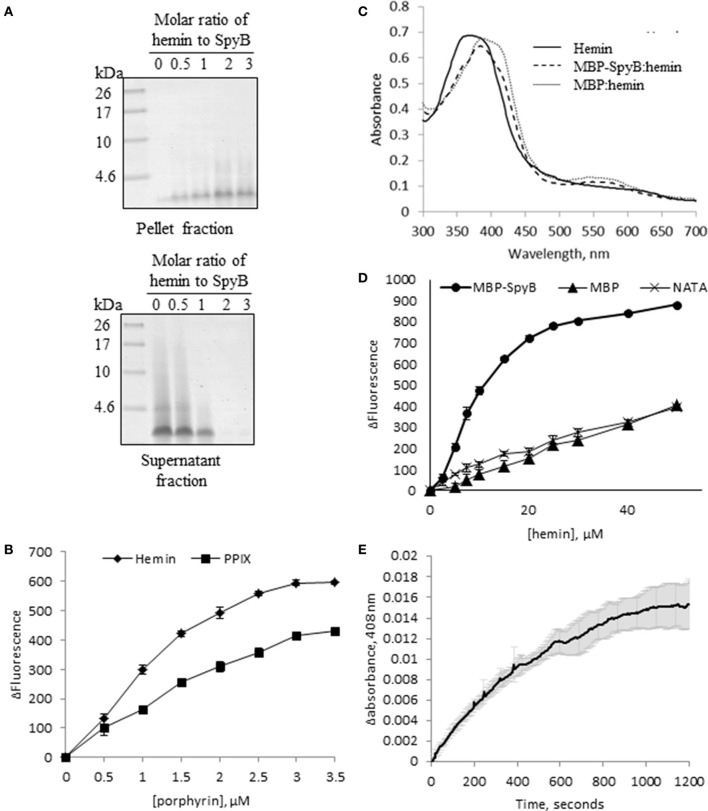
Figure 5.
SpyB is a porphyrin-binding protein. (A) Hemin binding decreases SpyB solubility. SpyB was mixed with hemin in different molar ratios. Soluble and insoluble fractions were separated by centrifugation and resolved on a 16% Tris-Tricine gel. (B) The change in tryptophan fluorescence quenching of SpyB by hemin and protoporphyrin IX (PPIX). A series of hemin or PPIX concentrations (0, 0.5, 1, 1.5, 2, 2.5, 3, and 3.5 μM) was added to 5 μM SpyB, in the presence of 1 mM DTT. The curves are the average of three replicates ± standard deviation. The dissociation constant (KD) was calculated using SigmaPlot software. (C) Absorbance spectra between 300 and 700 nm were collected for 50 μM hemin; 50 μM hemin mixed with 2.5 μM MBP-SpyB; and 50 μM hemin mixed with 2.5 μM MBP, in the presence of 1 mM DTT. (D) The change in tryptophan fluorescence quenching for MBP-SpyB and MBP. A series of hemin concentrations (0, 2.5, 5, 7.5, 10, 15, 20, 25, 30, 40, 50 μM) was added to 2.5 μM MBP-SpyB monomer or MBP, in the presence of 1 mM DTT. A control experiment was performed with 27.5 μM N-acetyltryptophanamide (NATA) under the same conditions. The curves are the average of three replicates ± standard deviation. The KD was calculated using SigmaPlot software. (E) A time course for hemin transfer from MBP-SpyB to apomyoglobin was measured at 408 nm over 20 min. The dissociation rate constant (Koff) was calculated from the change in absorbance at 408 nm using Graphpad Prisim software. Data are the average of three replicates ± standard deviation. The Koff along with the previously measured KD were used to calculate the binding rate constant (Kon) for MBP-SpyB.