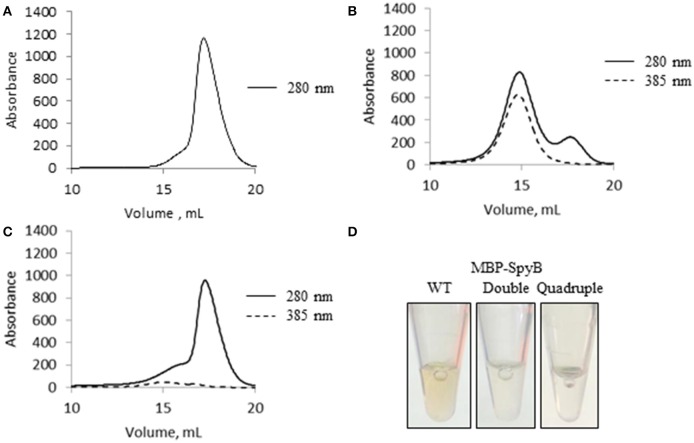
Figure 6.
Hemin binding induces SpyB oligomerization. (A) The size-exclusion chromatogram of MBP-SpyB monomer. (B) The size-exclusion chromatogram of MBP-SpyB monomer reconstituted with hemin in 1:4 ratio. (C) The size-exclusion chromatogram of MBP-SpyB monomer reconstituted with protoporphyrin IX in 1:4 ratio. The absorbance was monitored at 280 and 385 nm (hemin and protoporphyrin IX detection). (D) The appearance of WT MBP-SpyB, and double (C7A/C13A) and quadruple (C7A/C13A/C30A/C35A) MBP-SpyB mutants, following expression in E. coli Rosetta DE3 cells and purification by Ni-NTA affinity chromatography and size-exclusion chromatography. Data are representative of biological triplicates.