Abstract
Aims
It has been suggested that sulodexide is able to lower blood pressure (BP). This may be attributed to its ability to restore the endothelial surface layer (ESL). As ESL perturbation is known to be related to the degree of kidney damage, we investigated whether albuminuria, reflecting ESL status, modified the BP‐lowering potential of sulodexide.
Methods
A post hoc analysis of the double‐blind, randomized, placebo‐controlled sulodexide microalbuminuria (Sun‐MICRO) and macroalbuminuria (Sun‐MACRO) studies, including 1056 microalbuminuric and 843 macroalbuminuric subjects with type 2 diabetes receiving maximal tolerated renin–angiotensin‐aldosterone system inhibitor therapy, was carried out. We compared the effect of placebo and sulodexide on systolic BP (SBP) among albuminuria groups.
Results
Analysis of covariance, including data from both trials, showed that baseline urine albumin‐to‐creatinine ratio (UACR) was the only modifier of the SBP response (interaction with treatment P = 0.001). In subjects with an UACR >1000 mg g–1, sulodexide lowered SBP by 4.6 mmHg [95% confidence interval (CI) 3.6, 5.6; P < 0.001] compared with placebo, whereas a 2.3 mmHg (95% CI 0.9,3.7; P = 0.001) reduction was seen in subjects with a UACR of 300–1000 mg g–1. Sulodexide did not lower SBP in subjects with a UACR <300 mg g–1 (−0.2 mmHg, 95% CI −0.8, 0.5; P = 0.60). SBP‐lowering effects were not accompanied by changes in body weight.
Conclusion
The BP‐reducing potency of sulodexide is modified by the degree of albuminuria in subjects with type 2 diabetes. As ESL status deteriorates with increasing albuminuria and nephropathy severity, this suggests that ESL restoration may represent a new target for BP treatment in subjects with diabetic nephropathy.
Keywords: albuminuria, blood pressure, cardiovascular, glycosaminoglycan, hypertension, sulodexide
What is Already Known about this Subject
Sulodexide has been shown to have antihypertensive potency.
Sulodexide consists of glycosaminoglycans that are known to play an important role in endothelial function and sodium homeostasis.
There is a relationship between the volume of the endothelial surface layer and albuminuria.
What this Study Adds
The urine albumin–creatinine ratio is an important modifier of blood pressure responses to sulodexide treatment.
Sulodexide reduces blood pressure in macroalbuminuric diabetic subjects receiving maximally tolerated renin–angiotensin‐aldosterone system inhibitor therapy.
The endothelial surface layer, of which the status reflected by albuminuria levels, might represent a novel antihypertensive target in diabetes.
Table of Links
| TARGETS | |
|---|---|
| Enzymes 2 | GPCRs 3 |
| ACE | AT1‐receptor http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2534 |
This Table lists key protein targets in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 1 and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 2, 3.
Introduction
Approximately one billion people in the world suffer from hypertension 4. Despite effective antihypertensive treatment, half of the patients with hypertension have an uncontrolled blood pressure (BP) 5, 6, which has been shown to increase cardiovascular risk considerably 7. This number may be even higher in patients with diabetes, kidney disease or both. We therefore need new therapeutic inventions to improve BP control in these patients.
In this context, the endothelial surface layer (ESL) is of interest. The ESL is a dynamic layer that covers the inner surface of blood vessels throughout the body. The main constituents of the ESL are proteoglycans with attached glycosaminoglycans, glycoproteins and soluble proteins. The ESL has been shown to possess many vasoprotective properties as it improves endothelial barrier function, prevents leucocyte adhesion, possesses antithrombotic effects and preserves shear‐mediated nitric oxide production 8. In addition, endothelial glycosaminoglycans have been shown to be able to bind sodium, thereby providing an intravascular compartment for osmotically inactive sodium storage 9. A damaged ESL, which has been observed in conditions such as diabetes, chronic kidney disease and end‐stage renal disease, may therefore have significant implications for sodium homeostasis and BP regulation 10, 11, 12, 13. Consequently, ESL restoration may decrease BP. Sulodexide, an orally available mixture of glycosaminoglycans, has been shown to restore the ESL in diabetic patients 14. A meta‐analysis that gathered BP data from eight sulodexide trials, primarily investigating diabetic nephropathy patients, has shown that sulodexide also decreases BP 15. This was seen particularly in hypertensive patients, who showed placebo‐subtracted BP reductions that were identical to those found using current available antihypertensive drugs 15. Considering the vasoprotective properties of the ESL and its capacity to neutralize sodium‐driven effects on volume status by osmotic inactivation, this BP‐lowering effect seems to be attributed to ESL‐restoring properties. Sulodexide may therefore be particularly effective in conditions that are characterized by ESL damage.
Among diabetic patients, higher albuminuria levels are associated with a marked reduction in ESL volume 13. In the present post hoc analysis of the randomized, double‐blind, placebo‐controlled sulodexide microalbuminuria (Sun‐MICRO) and sulodexide macroalbuminuria (Sun‐MACRO) trials, we investigated whether the BP‐lowering effect of sulodexide is modified by the degree of albuminuria, in type 2 diabetic patients. Both trials were designed to investigate the potential renoprotective effect of sulodexide but did not show a reduction in albuminuria or in renal function decline 16, 17. The primary objective of the present analysis was to compare the BP‐lowering effects of sulodexide and placebo among albuminuria groups.
Methods
Subjects and study design
The Sun‐MICRO and Sun‐MACRO trials investigated the renoprotective effects of sulodexide 200 mg day–1 in subjects with type 2 diabetes and maximal tolerable renin–angiotensin–aldosterone system inhibitor therapy 16, 17, 18. The Sun‐MICRO trial included microalbuminuric subjects [a urine albumin‐to‐creatinine ratio (UACR) of 35–200 mg g–1 in men and 45–200 mg g–1 in women] with serum creatinine levels below 1.5 mg dl–1, whereas the Sun‐MACRO trial studied macroalbuminuric subjects (>0.9 g protein 24 h–1) with renal impairment (serum creatinine in women 1.3–3.0 mg dl–1; in men 1.5–3.0 mg dl–1). Other inclusion and exclusion criteria were similar between both trials. Both trials started simultaneously and were performed in the same geographical areas and research centres 18. As the rate of the primary renal endpoint, a composite of a doubling of baseline serum creatinine, development of end‐stage renal disease, or serum creatinine >6.0 mg dl–1, as well as proteinuria levels, was identical in placebo and sulodexide groups in an interim analysis of the Sun‐MACRO trial, the study was terminated early, after inclusion of 1248 subjects, with 1029 years of follow‐up. As a result of the early termination, the number of subjects that had available BP measurements decreased during the 6‐month (n = 702), 9‐month (n = 568) and 12‐month (n = 426) Sun‐MACRO follow‐up visits. The Sun‐MICRO trial was fully completed and the number of participants and BP recordings remained stable throughout the study. All participants gave written informed consent before enrolment in one of the studies. Both studies were approved by the appropriate local research ethics committee and performed in accordance with the Declaration of Helsinki of the World Medical Association. All data were processed anonymously.
Measurements
During both studies, at each visit, three seated BP measurements were obtained 1 min apart, after at least 10 min of rest. The mean BP of these measurements was used for analysis. BP was recorded at 2‐month intervals in the Sun‐MICRO trial and at 3‐month intervals in the Sun‐MACRO trial. The target BP in both trials was <130/80 mmHg. We calculated the individual BP changes from baseline to assess the effects of sulodexide and placebo. As most antihypertensive treatments exert their maximal BP‐reducing effects after 6–8 weeks, we used BP values from the first follow‐up visit to analyse whether UACR modified the observed BP effects (i.e. after 2 months or 3 months). In both trials, albuminuria was determined as the geometric mean of the ratio of urine albumin concentration to urine creatinine concentration for three consecutive first morning voided urine samples collected before the study visit.
Considering that the sodium‐binding potential of ESL glycosaminoglycans might serve as an explanation for sulodexide‐associated BP changes, we analysed changes in body weight to determine whether BP changes were related to changes in extracellular volume. In addition, we analysed plasma sodium concentrations during follow‐up, and investigated whether changes in plasma sodium concentrations or body weight were associated with BP changes. Plasma sodium concentration was measured only in the Sun‐MACRO study.
Statistics
Data are presented as means with standard deviations, or medians with interquartile ranges when skewed. We used independent sample t‐tests to compare BP reductions between treatment groups. To analyse whether the degree of albuminuria affected the BP effects of sulodexide, we used a one‐way analysis of covariance (ANCOVA), with treatment group as the fixed effect. The following patient characteristics were assessed as possible effect modifiers: age, gender, baseline systolic BP (SBP), diastolic BP (DBP), estimated glomerular filtration rate (eGFR), body mass index (BMI) and glycosylated haemoglobin (HbA1c). Baseline characteristics that were significantly associated with BP changes were entered in an ANCOVA model, along with UACR, to investigate whether these factors modified the BP response. For this analysis, UACR values were log transformed. Effect modification was assessed by the interaction term ‘treatment*patient characteristic’. To quantify the effects of albuminuria on the BP effects of sulodexide, we plotted the estimated SBP effects in a linear regression analysis and divided subjects in groups with an UACR of <300 mg g–1, 300–1000 mg g–1 and >1000 mg g–1.
To compare SBP, DBP, body weight and plasma sodium changes following sulodexide and placebo treatment during follow‐up, we used a general linear model for repeated measurements, with correction for baseline values. All statistical analyses were performed using SPSS (Version 21.0, SPSS, Inc., Chicago, IL, USA)
Results
Study population
BP measurements of 1056 and 843 subjects were available in the Sun‐MICRO and Sun‐MACRO trial, respectively. The average baseline BP was 131/74 mmHg in the Sun‐MICRO trial and 138/73 mmHg in the Sun‐MACRO trial. In comparison to the Sun‐MICRO trial, subjects included in the Sun‐MACRO trial had a lower eGFR and higher UACR at baseline (Table 1).
Table 1.
Baseline characteristics of patients included in the post hoc analysis
| Sun‐MICRO | Sun‐MACRO | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | All (n = 1056) | Placebo (n = 532) | Sulodexide (n = 524) | All (n = 843) | Placebo (n = 430) | Sulodexide (n = 413) |
| Male, n (%) | 802 (75.9) | 408 (76.7) | 394 (75.2) | 645 (76.3) | 320 (74.2) | 325 (78.5) |
| Age, years | 62.2 (9.8) | 62.3 (9.9) | 62 (9.7) | 62.9 (9.4) | 63.4 (9.6) | 62.3 (9.1) |
| SBP, mmHg | 131.2 (11.8) | 131.5 (11.8) | 130.9 (11.8) | 137.9 (14.3) | 137.6 (14.6) | 138.2 (14.0) |
| DBP, mmHg | 73.6 (11.9) | 73.4 (8.5) | 73.8 (8.7) | 73.2 (10.0) | 73.0 (10.1) | 73.4 (9.9) |
| BMI, kg m–2 | 32.1 (5.5) | 31.9 (5.3) | 32.3 (5.7) | 32.8 (15.5) | 31.7 (6.3) | 34 (21.3) |
| eGFR, ml min–1 1.73m–2 | 78.2 (22.4) | 77.9 (22.7) | 78.6 (22.1) | 33.4 (9.6) | 33.4 (9.7) | 33.4 (9.5) |
| UACR, mg g–1 median (IQR) | 96 (54–141) | 95 (57–143) | 97 (55–141) | 1348 (648–2363) | 1238 (606–2305) | 1431 (704–2401) |
| HbA1c, % | 7.6 (1.2) | 7.5 (1.3) | 7.6 (1.2) | 8.0 (1.6) | 7.9 (1.5) | 8.1 (1.7) |
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated haemoglobin; IQR, interquartile range; SBP, systolic blood pressure; SD, standard deviation; Sun‐MACRO, sulodexide macroalbuminuria; Sun‐MICRO, sulodexide microalbuminuria; UACR, urine albumin‐to‐creatinine ratio
BP effects during follow‐up
For the entire study population, placebo (1.4 ± 14.8 mmHg) and sulodexide (0.3 ± 14.8 mmHg) resulted in identical SBP changes (P = 0.10) after 2–3 months. In microalbuminuric subjects, sulodexide did not change BP relative to placebo. After 2 months, we observed comparable SBP changes in the sulodexide (1.3 ± 11.3 mmHg) and placebo (1.3 ± 11.7 mmHg) groups (placebo vs. sulodexide; P = 0.94). During the 26‐week follow‐up period in which the subjects remained on the study drug, SBP remained identical in both groups (Figure 1A). DBP was also similar in both groups during follow‐up. Interestingly, sulodexide‐ and placebo‐treated macroalbuminuric subjects showed an opposite SBP response after 3 months: SBP decreased in the sulodexide group by 0.9 ± 18.2 mmHg, while it increased in the placebo group by 1.7 ± 17.9 mmHg (placebo vs. sulodexide; P = 0.04). This SBP difference between sulodexide and placebo persisted after 6 months (−0.6 ± 17.8 mmHg vs. +2.3 ± 20.3 mmHg; P = 0.046). On average, SBP was 2.4 mmHg (P = 0.020) lower in the sulodexide group during the first year of treatment (Figure 1B). DBP did not differ between the groups.
Figure 1.

Sulodexide reduced systolic blood pressure (SBP) in patients with macroalbuminuria. Estimated marginal means (standard error of the mean) of SBP at baseline and after treatment with sulodexide and placebo in the sulodexide microalbuminuria (Sun‐MICRO) (A) and sulodexide macroalbuminuria (Sun‐MACRO) (B) trials. In patients with microalbuminuria, SBP was identical in both groups (P = 0.88). In macroalbuminuric patients, SBP was, on average, 2.4 mmHg lower [95% confidence interval (CI) 0.4, 4.4; P = 0.020) in the sulodexide group (n = 204) when compared with placebo (n = 198). We used a general linear model repeated measures test, with correction for baseline SBP for calculations
BP effect determinants
As albuminuria levels correlate with ESL status and may therefore affect the BP response to sulodexide, we analysed whether SBP changes were modified by baseline UACR. We observed a significant interaction between treatment and baseline UACR (P = 0.001), indicating that the SBP effects of sulodexide were modified by baseline BP (Table 2, Figure 2A). Other potential characteristics, including SBP (P = 0.98 for interaction) and DBP (P = 0.93 for interaction), did not show an interaction with treatment. The interaction between baseline UACR and treatment remained significant after adjustment for trial, baseline age, gender, eGFR, BMI and HbA1c. Consequently, the largest SBP changes between the placebo and sulodexide groups were observed in subjects with the highest degree of albuminuria. Subjects with an UACR >1000 mg g–1 who received sulodexide treatment had a 4.6 mmHg [95% confidence interval (CI) 3.6, 5.6; P < 0.001] lower SBP than the placebo‐treated subjects (Figure 2B). In subjects with an UACR between 300 mg g–1 and 1000 mg g–1, SBP was, on average, 2.3 mmHg (95% CI: 0.9, 3.7; P = 0.001) lower in the sulodexide group (P = 0.001). Microalbuminuric (<300 mg g–1) patients had similar BP changes following sulodexide and placebo treatment in this combined analysis of both studies (0.2 mmHg, 95% CI −0.5, 0.8; P = 0.60; Figure 2B).
Table 2.
Effect of urine albumin‐to‐creatinine ratio (UACR) on blood pressure (BP) changes induced by sulodexide. The results of the analysis of covariance model that was used to investigate whether baseline BP or UACR significantly affected the systolic BP (SBP) change that was observed after sulodexide or placebo treatment (upper five rows with green background). To test whether baseline BP or UACR modified the SBP change in placebo and sulodexide groups differently, we tested for an interaction between the included patient characteristics and treatment (lower three rows with white background). We observed a significant interaction for baseline UACR, but not for SBP or diastolic BP (DBP), indicating that the BP effects of sulodexide are modified by baseline UACR only. The represented values for treatment, trial, and baseline UACR, SBP and DBP were calculated without taking into account the effect of any interaction. The significance of each interaction was calculated separately
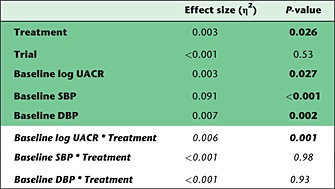
Figure 2.

The blood pressure (BP)‐lowering potential of sulodexide depends on albuminuria severity. (A) Regression lines and 95% confidence intervals (CIs) of the analysis of covariance (ANCOVA) demonstrating the significant interaction (P = 0.001) between the baseline urine albumin‐to‐creatinine ratio (UACR) and treatment arms (placebo vs. sulodexide). The regression line slopes of placebo (P < 0.001) and sulodexide (P < 0.001) were both significantly different from zero. (B) Quantitative analysis of the results of the ANCOVA, showing that subjects with an UACR >1000 mg g–1 benefit most from sulodexide treatment in terms of BP (mean and 95% CI). Sulodexide resulted in a 2.0 mmHg (95% CI −2.6, −1.3) systolic BP (SBP) reduction, while placebo increased SBP by 2.5 mmHg (95% CI 1.9, 3.3). In the group with an UACR between 300–1000 mg g–1, sulodexide decreased BP by 0.8 mmHg (95% CI −1.8, 0.2) and placebo increased BP by 1.6 mmHg (95% CI 0.6, 2.5), while subjects with an UACR <300 mg g–1 had an identical BP response (0.2 mmHg, 95% CI −0.5, 0.8; P = 0.60)
As severe proteinuria may be a reflection of podocytopathy rather than additional damage to the endothelium and ESL, we analysed the SBP changes in subgroups of patients with an UACR >1000 mg g–1. Although the difference in SBP change remained significant between placebo and sulodexide in subgroups with an UACR of 1000–2000, 2000–3000 and >3000, the SBP changes were identical among these subgroups for placebo (P = 0.38) and sulodexide (P = 0.66). To test the robustness of our results, we analysed the Sun‐MICRO and Sun‐MACRO study separately. This analysis demonstrated similar results, showing that baseline UACR was not a determinant of SBP change in Sun‐MICRO, whereas a significant interaction between treatment and UACR was demonstrated in Sun‐MACRO (data not shown).
Plasma sodium and body weight
To explore whether sulodexide affected extracellular volume regulation by osmotic inactivation of sodium, we analysed plasma sodium concentration and body weight. Body weight remained identical in sulodexide‐ and placebo‐treated subjects from Sun‐MICRO during follow‐up (P = 0.12). In the Sun‐MACRO study, plasma sodium concentrations decreased in the placebo (−0.3 ± 2.9 mmol l–1; P = 0.03) and sulodexide (−0.6 ± 3.0 mmol l–1; P < 0.001) groups to a similar extent after 3 months (P = 0.07). During the same period, plasma glucose increased similarly after placebo (1.0 ± 5.0 mmol l–1; P < 0.001) and sulodexide treatment (0.7 ± 4.8 mmol l–1; P = 0.001). Similar to the results in microalbuminuric subjects, body weight remained similar in macroalbuminuric subjects during follow‐up (P = 0.83).
Discussion
The present post hoc analysis demonstrated that the BP‐reducing potency of sulodexide depends on nephropathy severity in subjects with type 2 diabetes and maximum tolerated renin–angiotensin‐aldosterone system inhibitor therapy. Relative to placebo treatment, the largest BP reductions after sulodexide treatment were seen in subjects with the highest degree of albuminuria (>1000 mg g–1), who showed an additional 5 mmHg SBP reduction, despite a well‐controlled average BP at baseline. In microalbuminuric patients (UACR <300 mg g–1), sulodexide and placebo had similar effects on SBP. Considering the high cardiovascular risk of patients with severe diabetic nephropathy, it is likely that the observed added BP‐lowering effect of sulodexide translates into a further reduction of cardiovascular risk.
A meta‐analysis of randomized controlled trials that investigated sulodexide has recently reported on the so‐far overlooked systemic BP effects of sulodexide 15. In this previous analysis, in which albuminuria could not be studied, baseline BP was identified as the most important determinant of the observed BP response. In the current post hoc analysis, we were able to analyse individual patient data, including albuminuria. However, we could not reproduce this finding using data from two large trials, in which average BP was well controlled. Although baseline SBP was the most important factor affecting SBP responses, we observed similar associations for placebo and sulodexide treatment groups. This indicates that the association between baseline BP and the SBP response is most likely caused by a regression‐to‐the‐mean phenomenon. As the inclusion BP was 150/90 mmHg and 160/100 mmHg in the Sun‐MICRO and Sun‐MACRO studies, respectively, we were not able to draw any conclusions for BP values above these levels. The most likely explanation for the absent DBP response after sulodexide treatment is the low baseline DBP.
Albuminuria is known to be a sign of kidney damage but may also represent systemic vascular disease 19. In this respect, the ESL is an interesting vascular entity as it is involved in both systemic vascular function and glomerular permeability (i.e. albuminuria). In rats, for example, mesenteric and vascular ESL thicknesses were both negatively correlated with proteinuria 20. This relationship has also been observed in humans. In subjects with type 1 diabetes, the presence of albuminuria was associated with a 75% reduction in systemic ESL volume in comparison with nonalbuminuric controls 13. These studies suggest that albuminuria reflects systemic ESL damage. As sulodexide is known to restore a damaged ESL in diabetic patients, one may therefore expect that subjects with a higher degree of albuminuria (i.e. a smaller systemic ESL volume) may benefit most from sulodexide therapy 14. This is in line with the results of the present post hoc analysis, in which subjects with a higher degree of albuminuria, who may benefit most from sulodexide in terms of ESL restoration, showed the largest BP reductions. However, proteinuria levels above 1 g are generally thought to be caused by podocyte injury rather than by additional glomerular ESL damage. As the BP‐lowering effects of sulodexide may be ascribed to ESL restoration, BP may not decrease any further under conditions of severe proteinuria. Indeed, in our subgroup analysis of subjects with UACR levels >1000 mg g–1, we observed that SBP changes between placebo and sulodexide remained significant but did not increase further.
The BP‐lowering potential of sulodexide may be explained by several mechanisms, all of which can be attributed to an increased ESL volume. Firstly, the ESL is an important mediator of shear‐mediated nitric oxide production. Secondly, ESL glycosaminoglycans have been shown to be able to bind to and osmotically inactivate sodium, thereby reducing the amount of osmotically active sodium, which prevents water retention and a subsequent BP increase 9, 10, 21. Both mechanisms may be responsible for the observation that the largest BP reductions after sulodexide treatment were seen in subjects with the highest degree of albuminuria. The observed reduction in BP was not characterized by any change in body weight, suggesting that extracellular volume remained identical. However, plasma sodium concentration decreased twice as much after sulodexide treatment than after placebo, albeit not significantly. Although the 0.6 mmol l–1 decrease may seem marginal, it is similar to reductions in plasma sodium concentration achieved with long‐term modest salt reduction 22. The absence of a weight change, together with the decrease in plasma sodium concentration that was seen alongside the BP reduction, indicates that sulodexide‐treated patients had less osmotically active sodium per litre of body water. This suggests that sulodexide is able to increase the capacity for non‐osmotic sodium storage. As these studies lacked data on extracellular volume or total body water, these results should be interpreted cautiously, and future studies should investigate whether sulodexide interferes with sodium and volume homeostasis.
Besides albuminuria, plasma glucose has been reported to affect ESL status 23. In the present analysis, however, we were not able to demonstrate an association between plasma glucose and SBP response, either in micro‐ or macroalbuminuric subjects. This may be explained by the fact that a previous study observed differences in ESL status while examining large intraindividual plasma glucose differences, whereas we assessed small interindividual differences in the present study. In addition, eGFR has been shown to affect ESL volume 11 but was not associated with the SBP response in the present study. However, Padberg et al. observed a similar association between both syndecan‐1, an ESL‐shedding product, and proteinuria as well as between syndecan‐1 and eGFR 11. As a decrease in eGFR and development of albuminuria are both hallmarks of diabetic nephropathy, it is difficult to assess which factor may be responsible for the reduction in ESL volume.
Similar to the previously mentioned meta‐analysis, the present post hoc analysis suggests that future studies should take the systemic BP effects into account when analysing the potential renoprotective effects of sulodexide 15. In both analyses, changes in BP and in UACR showed a significant correlation with a regression line that had a y‐intercept similar to zero.
The strength of the present study was the availability of individual patient data from two large randomized placebo‐controlled trials 24. To cover all ranges of albuminuria, we pooled the data from the Sun‐MICRO and Sun‐MACRO trials to investigate whether baseline albuminuria modified the SBP responses. Although both trials were comparable with regard to design, participating centres and measurements, possible differences between studies could have induced bias. However, we observed similar results in separate analyses of the Sun‐MICRO and Sun‐MACRO trials. In addition, we included trial as a covariate in the regression analysis, which did not appear to have a significant influence. A potential limitation of the present analysis was the lack of 24 h BP data, which may have been of additive value. Another potential limitation was that patients were permitted to use adjunctive antihypertensive agents. However, as both micro‐ and macroalbuminuric subjects were allowed to take additional agents, and both trials were double blinded, this effect was not likely to influence our results significantly. Due to the nature of a post hoc analysis, we were not able to prove causality, so these results should be interpreted cautiously.
Conclusion
The present post hoc analysis of the randomized, double‐blind, placebo‐controlled Sun‐MICRO and Sun‐MACRO trials showed that the BP‐reducing potency of sulodexide is modified by the degree of albuminuria in type 2 diabetic patients. Although the average subject was normotensive, sulodexide reduced SBP by 5 mmHg in diabetic subjects with the highest degree of albuminuria, indicating that sulodexide may help to control BP in patients with severe diabetic nephropathy. As the degree of albuminuria is generally believed to relate to ESL damage, our findings indicate that ESL restoration may represent a new target for BP treatment.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; DdZ was a member of the advisory board of Keryx Biopharmaceuticals in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
LV was supported by Kolff Grant KJPB 11.22 from the Dutch Kidney Foundation and Clinical Fellowship Grant 90700310 from The Netherlands Organization for Scientific Research.
Olde Engberink, R. H. G. , Heerspink, H. J. L. , de Zeeuw, D. , and Vogt, L. (2016) Blood pressure‐lowering effects of sulodexide depend on albuminuria severity: post hoc analysis of the sulodexide microalbuminuria and macroalbuminuria studies. Br J Clin Pharmacol, 82: 1351–1357. doi: 10.1111/bcp.13062.
References
- 1. Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 2016; 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 2015; 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE, et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 2015; 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure). National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country‐years and 5.4 million participants. Lancet 2011; 377: 568–577. [DOI] [PubMed] [Google Scholar]
- 5. Egan BM, Li J, Qanungo S, Wolfman TE. Blood pressure and cholesterol control in hypertensive hypercholesterolemic patients: national health and nutrition examination surveys 1988–2010. Circulation 2013; 128: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Center for Disease Controle and Prevention (CDC) . Vital signs: prevalence, treatment, and control of hypertension – United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep 2011; 60: 103–108. [PubMed] [Google Scholar]
- 7. Blacher J, Evans A, Arveiler D, Amouyel P, Ferrieres J, Bingham A, et al. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study. J Hum Hypertens 2010; 24: 19–26. [DOI] [PubMed] [Google Scholar]
- 8. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 2007; 454: 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siegel G, Walter A, Kauschmann A, Malmsten M, Buddecke E. Anionic biopolymers as blood flow sensors. Biosens Bioelectron 1996; 11: 281–294. [DOI] [PubMed] [Google Scholar]
- 10. Olde Engberink RH, Rorije NM, Homan van der Heide JJ, van den Born BJ, Vogt L. Role of the vascular wall in sodium homeostasis and salt sensitivity. J Am Soc Nephrol 2015; 26: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis 2014; 234: 335–343. [DOI] [PubMed] [Google Scholar]
- 12. Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol 2012; 23: 1900–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 2006; 55: 1127–1132. [DOI] [PubMed] [Google Scholar]
- 14. Broekhuizen LN, Lemkes BA, Mooij HL, Meuwese MC, Verberne H, Holleman F, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia 2010; 53: 2646–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olde Engberink RH, Rorije NM, Lambers Heerspink HJ, De Zeeuw D, van den Born BJ, Vogt L. The blood pressure lowering potential of sulodexide – a systematic review and meta‐analysis. Br J Clin Pharmacol 2015; 80: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis EJ, Lewis JB, Greene T, Hunsicker LG, Berl T, Pohl MA, et al. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trial. Am J Kidney Dis 2011; 58: 729–736. [DOI] [PubMed] [Google Scholar]
- 17. Packham DK, Wolfe R, Reutens AT, Berl T, Heerspink HL, Rohde R, et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol 2012; 23: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambers Heerspink HJ, Fowler MJ, Volgi J, Reutens AT, Klein I, Herskovits TA, et al. Rationale for and study design of the sulodexide trials in type 2 diabetic, hypertensive patients with microalbuminuria or overt nephropathy. Diabet Med 2007; 24: 1290–1295. [DOI] [PubMed] [Google Scholar]
- 19. Weir MR. Microalbuminuria and cardiovascular disease. Clin J Am Soc Nephrol 2007; 2: 581–590. [DOI] [PubMed] [Google Scholar]
- 20. Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol 2012; 23: 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farber SJ, Schubert M, Schuster N. The binding of cations by chondroitin sulfate. J Clin Invest 1957; 36: 1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension 2005; 45: 98–102. [DOI] [PubMed] [Google Scholar]
- 23. Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo . Diabetes 2006; 55: 480–486. [DOI] [PubMed] [Google Scholar]
- 24. Riley RD, Lambert PC, Abo‐Zaid G. Meta‐analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010; 340: c221. [DOI] [PubMed] [Google Scholar]


