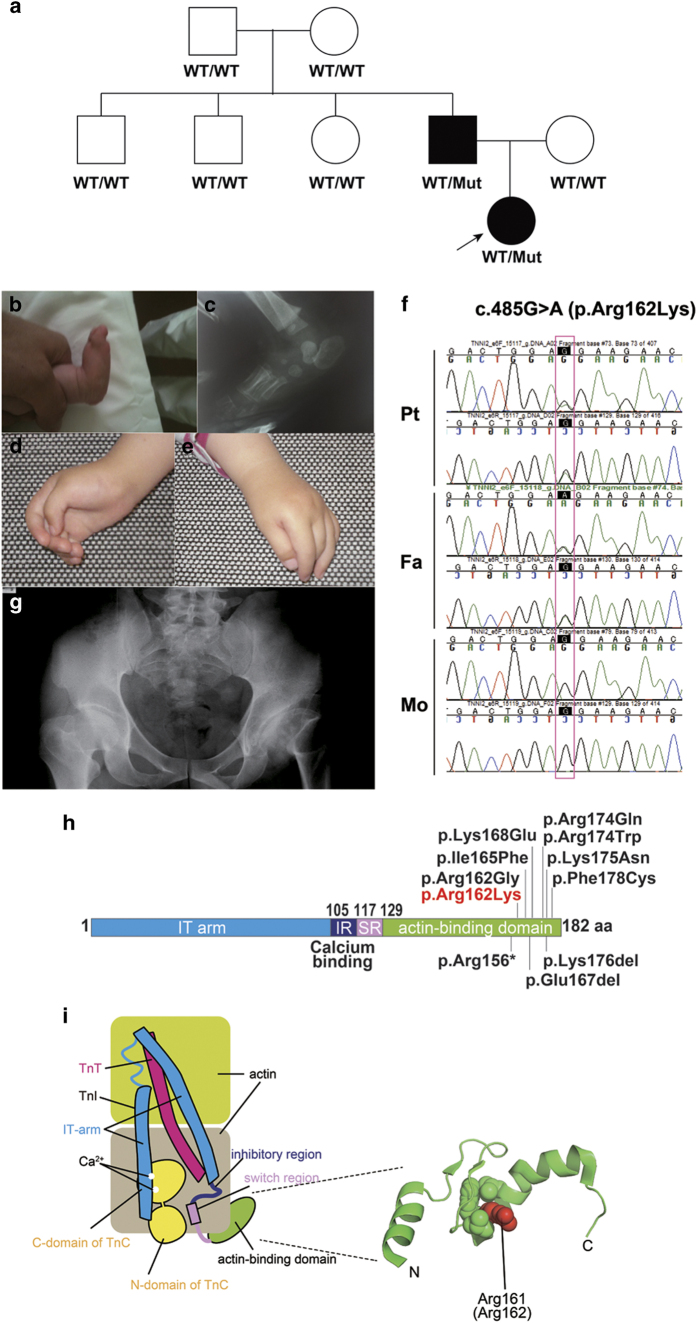
Figure 1.
(a) Pedigree tree and segregation status of TNNI2 mutation (c.485G>A, p.Arg162Lys). WT: wild type, Mut: mutant (b–e) The female patient showing vertical talus (b, c) and overlapping fingers (d, e). Electropherograms around the mutation of the patient and her parents (f). The father had hip dysplasia (g). TNNI2 mutations were mapped to the protein (h). Functional domains are colored differently (ID: inhibitory region, SR: switch region). The current (red) and reported (black) mutations were clustered in the actin-binding domain (h). Schematic representation of the troponin complex with an actin polymer in low Ca2+ concentrations, modified from Murakami et al. (i).13 The Ca2+-binding (TnC) and tropomyosin-binding (TnT) subunits are depicted as a yellow dumbbell shape and a magenta tube, respectively. The domain organization of the inhibitory subunit (TnI)14 is indicated; the IT-arm, which comprises two helices connected by a flexible linker, the inhibitory region, the switch region and the C-terminal actin-binding domain, is shown as cyan tube-like shapes connected with a cyan string, a dark-blue string, a pink rectangle and string, and a green oval, respectively. Actin monomers are presented as colored rounded rectangles. The mutation position of human TnI was mapped in a solution of the actin-binding domain of chicken TnI in a Ca2+-free state (PDB code 1VDI).13 The mutation site is colored red. The side chains of the residue at the mutation site (Arg161 in chicken) and its surrounding residues are shown as van der Waals spheres. The amino acid number of chicken TnI is shown with that of human TnI in parentheses. N and C denote the N- and C-terminus of the protein.