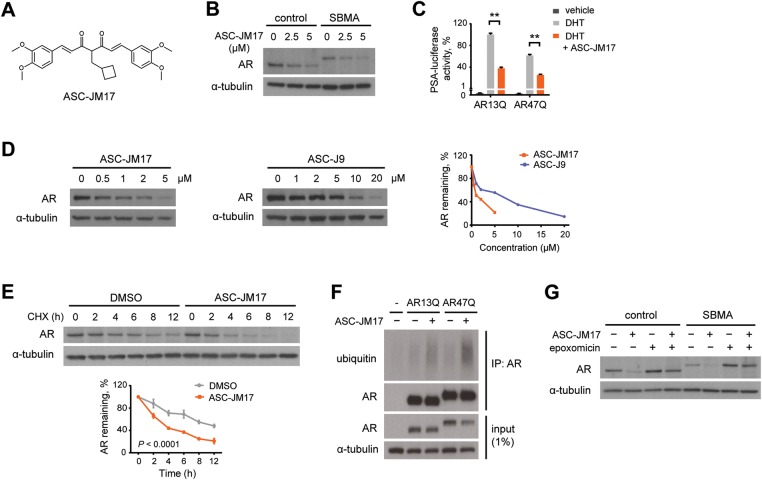
Figure 1.
ASC-JM17 reduces AR protein through enhanced degradation by the UPS. (A) Chemical structure of ASC-JM17. (B) Effect of ASC-JM17 on AR protein levels. Fibroblasts derived from a healthy control and an SBMA patient with 68 CAG repeats were treated with 10 nm DHT and indicated concentrations of ASC-JM17, and analyzed by western blotting. (C) Effect of ASC-JM17 on AR transcriptional activity. PC12 cells expressing AR with wild-type (AR13Q) or expanded (AR47Q) polyglutamine tracts were incubated with ethanol vehicle or DHT (10 nm), with and without 5 µm ASC-JM17. PSA-luciferase activity was measured using the dual luciferase assay (n = 3). Data are expressed as mean ± SEM. **P < 0.01 (two-way ANOVA). (D) Patient fibroblasts were treated with indicated concentrations of ASC-JM17 or ASC-J9 and analyzed by western blotting. AR band intensities were quantified and normalized to α-tubulin. (E) Patient fibroblasts were treated with 10 µm cycloheximide (CHX), with or without 5 µm ASC-JM17 and harvested at indicated time points. The graph shows quantification of AR band intensities normalized to α-tubulin (n = 3). Data are expressed as mean ± SEM (two-way ANOVA). (F) PC12 cells transfected with AR13Q, AR47Q or empty vector were treated with 5 µm ASC-JM17 or DMSO vehicle. AR was immunoprecipitated from whole-cell extracts and its ubiquitylation status was assessed using the ubiquitin antibody FK2. (G) Fibroblasts were incubated with 5 µm ASC-JM17 in the presence or absence of 100 nm epoxomicin.