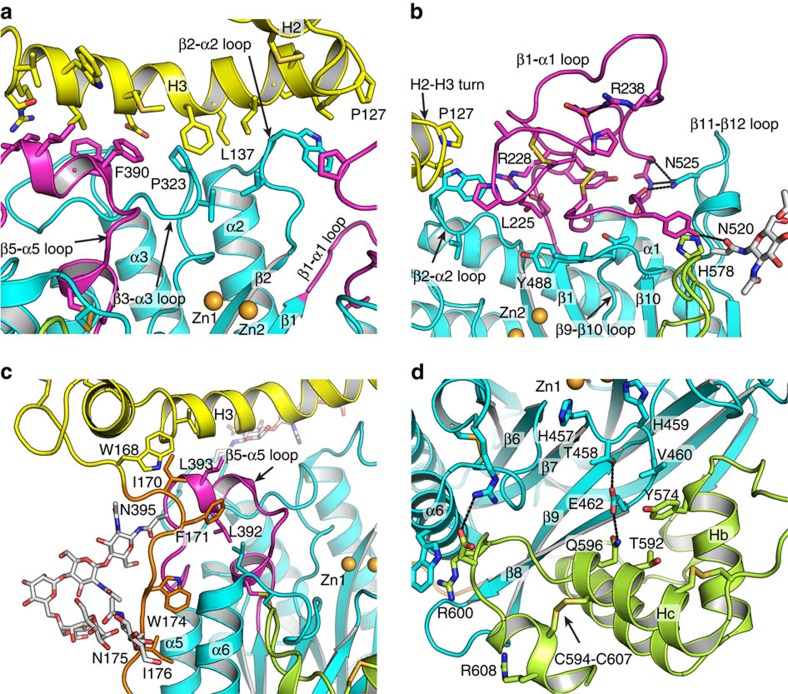
Figure 2. Structural details of the substrate binding cleft.
(a) Interface between H3 in saposin and the β5-α5, β3-α3, β2-α2 loops in the catalytic domain. (b) β1-α1 loop in catalytic domain near by the tip of H2-H3 turn in saposin domain. (c) Hydrophobic interactions between N-terminal part of the proline-rich linker and catalytic domain. (d) Interface between the C-terminal domain and catalytic domain.