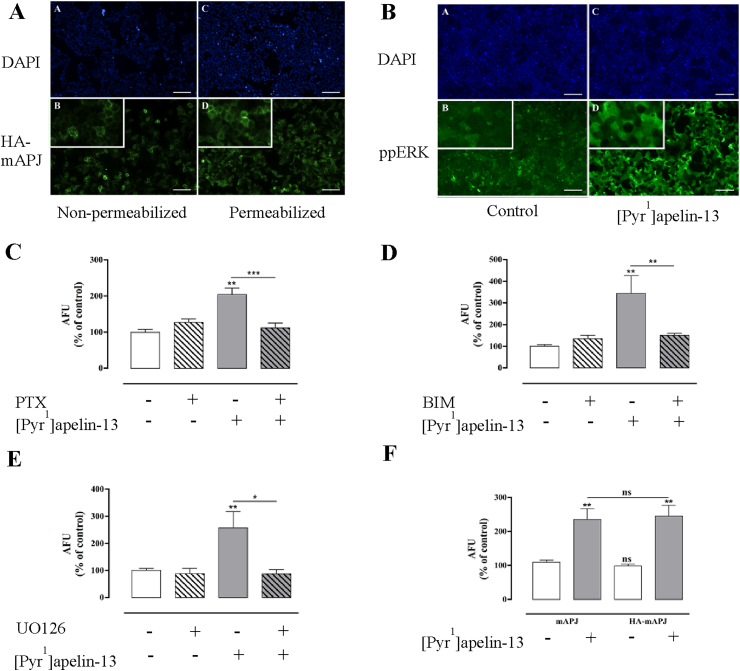
Fig. 1.
(A) Thumbnail images from individual wells stained for HA-mAPJ cell surface and whole cell expression, after stimulation with [Pyr1]apelin-13. Representative regions of cell images are shown for DAPI (top panels A and C), and HA-mAPJ (bottom panels B and D) in HA-mAPJ-HEK293 cells with either non-permeabilized (left panels, cell surface) or permeabilized (right panels, whole cell) membranes, higher magnification inset. (B) Thumbnail images from individual wells stained for ppERK1/2 expression after stimulation with [Pyr1]apelin-13. Representative regions of cell images are shown for DAPI (top panels A and C), and ppERK1/2 (bottom panels B and D) in mAPJ-HEK293 cells stimulated with either vehicle control (left panels) or 100 nM [Pyr1]apelin-13 (right panels), higher magnification inset. Scale bars, 100 μm. mAPJ-HEK293 cells were pre-treated with or without (C) PTX (200 ng/ml, 16 h), (D) BIM (10 μM, 1 h) or (E) UO126 (10 μM, 30 min) and stimulated in the presence or absence of [Pyr1]apelin-13 (100 nM) for 5 min. (F) Tagging of mAPJ with the HA epitope did not interfere with receptor signalling. Non tagged mAPJ-HEK293 cells and HA-tagged mAPJ-HEK293 cells were stimulated with [Pyr1]apelin-13 (100 nM) for 5 min and compared with control cells treated with 1× PBS. For (C–F) cells were fixed, stained, and imaged for determination of whole-cell ppERK1/2 intensity using anti ppERK1/2 antibody. The value determined with no primary antibody present was designated as background and was subtracted from raw data to give arbitrary fluorescence units (AFU) and then normalized to a percentage of vehicle control. Data shown are mean ± SEM, of at least three separate experiments, each with triplicate wells and triplicate fields within wells. *p < 0.05, **p < 0.01, and ***p < 0.001 comparing stimulations to basal conditions, analysed by one-way ANOVA and Dunnett's multiple comparison post hoc tests. ns = no statistical significant difference.