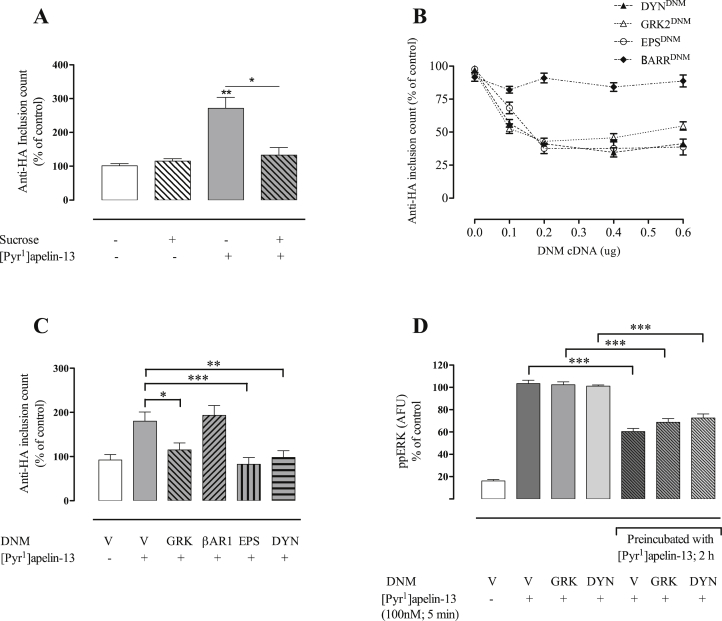
Fig. 6.
Mechanisms of [Pyr1]apelin-13-induced HA-mAPJ internalization and mAPJ desensitization. (A) HA-mAPJ-HEK293 cells were pre-treated with anti-HA antibody (1:100; 1 h, 37 °C/5% CO2), washed, then incubated in the presence or absence of sucrose (0.4 M) for 45 min, followed by incubation with [Pyr1]apelin-13 (100 nM) for 2 h. (B, C) HA-mAPJ-HEK293 cells were transfected with DNM cDNAs of various CME-related factors or an empty plasmid vector (V). After 48 h, cells were pre-treated with anti-HA antibody (1:100; 1 h, 37 °C/5% CO2), washed, incubated in the presence or absence of [Pyr1]apelin-13 (100 nM), for 2 h. Cells were fixed, stained and imaged for determination of whole cell inclusion counts, normalized to a percentage of vehicle control. (B) shows [Pyr1]apelin-13-induced HA-mAPJ internalization after co-transfection with increasing concentrations of GRKDNM, EPSDNM, DYNDNM and βARRDNM. (C) shows the effects of GRKDNM, EPSDNM, DYNDNM and βARRDNM on [Pyr1]apelin-13-induced HA-mAPJ internalization. In (D) mAPJ-HEK293 cells were transfected with DNM cDNAs of various CME-related factors or an empty plasmid vector (V). After 48 h, cells were washed, incubated in the presence or absence of [Pyr1]apelin-13 (100 nM), for 2 h, stimulated with 100 nM [Pyr1]apelin-13 for 5 min, fixed, stained and imaged for determination of whole cell ppERK1/2 intensity using anti-ppERK1/2 antibody, expressed as arbitrary fluorescent units (AFU). Data shown are mean ± SEM, of at least three separate experiments, each with triplicate wells and triplicate fields within wells. *p < 0.05, **p < 0.01, ***p < 0.001 analysed by two-way ANOVA and Dunnett's multiple comparison post hoc tests.