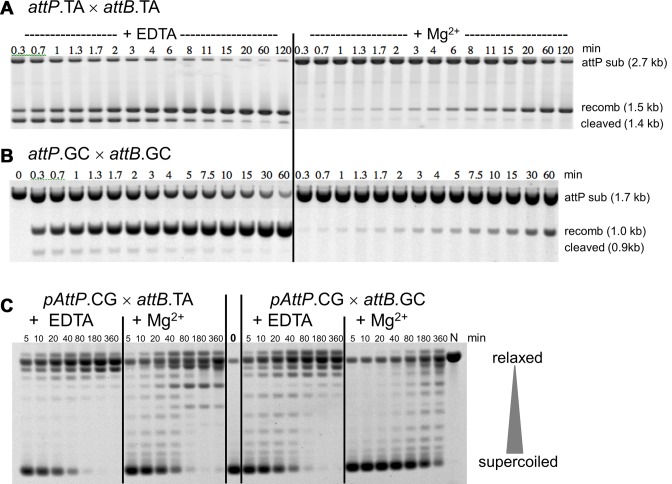
Figure 2.
Kinetic analysis of substrate cleavage and supercoil relaxation by Bxb1 Int in ensemble reactions. (A) A 2.7 kb linear substrate containing attP.TA at its center was reacted with Int and a 312 bp attB.TA in the absence or presence of Mg2+ for the times indicated and the products were analyzed by agarose gel electrophoresis. The two attP cleavage products (both 1.36 kb) migrate as a single band, as do the two recombinant products (1.52 kb). The fast-migrating attB bands have been omitted. (B) Similar experiments with a 1.7 kb linear attP.GC and a 312 bp attB.GC, showing the cleavage and recombinant products of 860 bp and 1.0 kb respectively. (C) A supercoiled plasmid with attP.CG was reacted with mismatched (and recombination-incompatible) 312 bp attB substrates containing either attB.TA or attB.GC for the times indicated, in the absence or presence of Mg2+. Agarose gel electrophoresis reveals the extent of plasmid relaxation: more relaxed topological isomers migrate slower than those with more supercoils. The lanes marked 0 and N show the unreacted and nicked forms of the pAttP.GC substrate; the position of linearized substrate is indicated. As supercoils are relaxed their migration rate is reduced, with the most relaxed forms running close to the nicked marker. However, because the precise helical parameters of DNA are influenced by presence or absence of Mg2+ and univalent ionic conditions, the most relaxed (but covalently closed) species migrate at positions that differ from each other and from that of the nicked marker (N). Thus, the most relaxed species formed in the presence of Mg2+ behave as slightly positively supercoiled in the gel and form the 2–3 bands clearly visible in the later lanes of the reactions with Mg2+ that migrate ahead of the nicked marker. See Supplementary Figure S1 for an alternative image of these data.