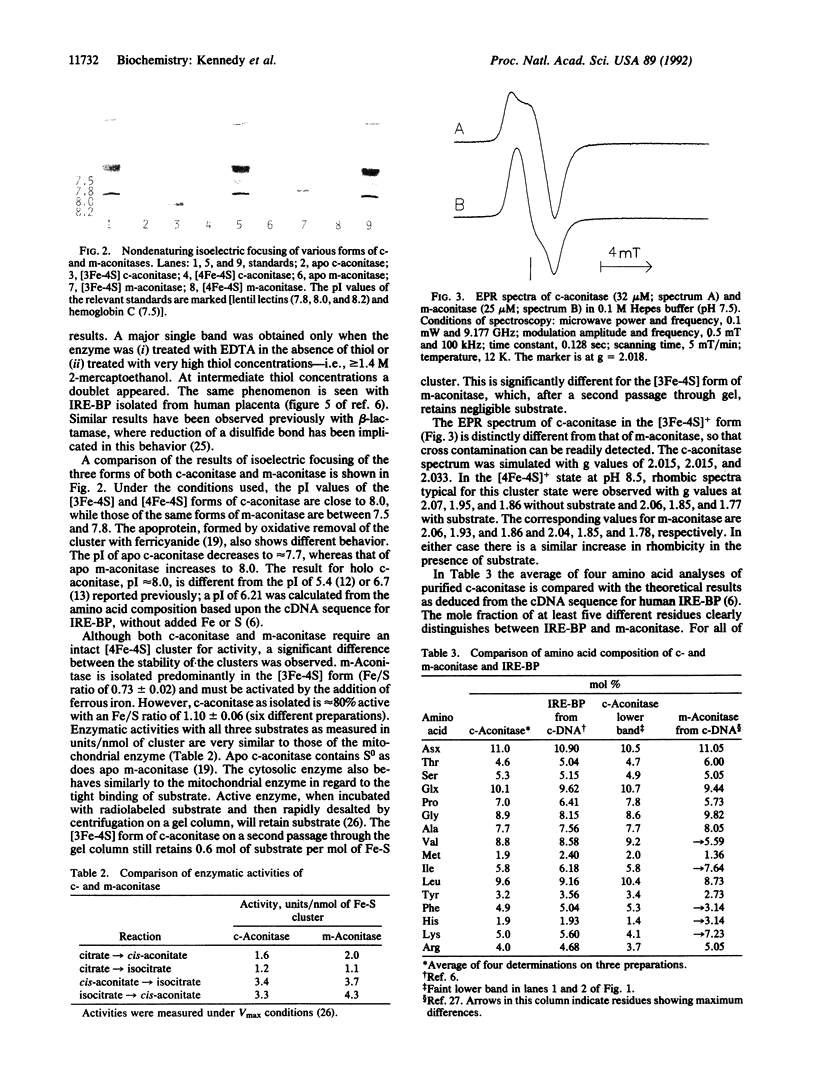
Abstract
In recent reports attention has been drawn to the extensive amino acid homology between pig heart, yeast, and Escherichia coli aconitases (EC 4.2.1.3) and the iron-responsive element binding protein (IRE-BP) of mammalian cells [Rouault, T. A., Stout, C. D., Kaptain, S., Harford, J. B. & Klausner, R. D. (1991) Cell 64, 881-883.; Hentze, M. W. & Argos, P. (1991) Nucleic Acids Res. 19, 1739-1740.; Prodromou, C., Artymiuk, P. J. & Guest, J. R. (1992) Eur. J. Biochem. 204, 599-609]. Iron-responsive elements (IREs) are stem-loop structures located in the untranslated regions of mRNAs. IRE-BP is required in the posttranscriptional regulation of ferritin mRNA translation and stabilization of transferrin receptor mRNA. In spite of substantial homology between the amino acid sequences of mammalian mitochondrial aconitase and IRE-BP, the mitochondrial protein does not bind IREs. However, there is a second aconitase, found only in the cytosol of mammalian tissues, that might serve as an IRE-BP. To test this possibility, we have prepared sufficient quantities of the heretofore poorly characterized beef liver cytosolic aconitase. This enzyme is isolated largely in its active [4Fe-4S] form and has a turnover number similar to that of mitochondrial aconitase. The EPR spectra of the two enzymes are markedly different. The amino acid composition, molecular weight, isoelectric point, and the sequences of six random peptides clearly show that these physicochemical and structural characteristics are identical to those of IRE-BP, and that c-aconitase is distinctly different from m-aconitase. In addition, both cytosolic aconitase and IRE-BP can have aconitase activity or function as IRE-BPs, as shown in the following paper and elsewhere [Zheng, L. Kennedy, M. C., Blondin, G. A., Beinert, H. & Zalkin, H. (1992) Arch. Biochem. Biophys., in press]. This leads us to the conclusion that cytosolic aconitase is IRE-BP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRANE F. L., GLENN J. L., GREEN D. E. Studies on the electron transfer system. IV. The electron transfer particle. Biochim Biophys Acta. 1956 Dec;22(3):475–487. doi: 10.1016/0006-3002(56)90058-0. [DOI] [PubMed] [Google Scholar]
- Eanes R. Z., Kun E. Inhibition of liver aconitase isozymes by (-)-erythro-fluorocitrate. Mol Pharmacol. 1974 Jan;10(1):130–139. [PubMed] [Google Scholar]
- Guarriero-Bobyleva V., Volpi-Becchi M. A., Masini A. Parallel partial purification of cytoplasmic and mitochondrial aconitate hydratases from rat liver. Eur J Biochem. 1973 May 2;34(3):455–458. doi: 10.1111/j.1432-1033.1973.tb02779.x. [DOI] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D. Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11735–11739. doi: 10.1073/pnas.89.24.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7536–7540. doi: 10.1073/pnas.89.16.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson C. P., Cleland W. W. Purification and kinetic studies of beef liver cytoplasmic aconitase. J Biol Chem. 1967 Sep 10;242(17):3833–3838. [PubMed] [Google Scholar]
- Hentze M. W., Argos P. Homology between IRE-BP, a regulatory RNA-binding protein, aconitase, and isopropylmalate isomerase. Nucleic Acids Res. 1991 Apr 25;19(8):1739–1740. doi: 10.1093/nar/19.8.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirling H., Emery-Goodman A., Thompson N., Neupert B., Seiser C., Kühn L. C. Expression of active iron regulatory factor from a full-length human cDNA by in vitro transcription/translation. Nucleic Acids Res. 1992 Jan 11;20(1):33–39. doi: 10.1093/nar/20.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptain S., Downey W. E., Tang C., Philpott C., Haile D., Orloff D. G., Harford J. B., Rouault T. A., Klausner R. D. A regulated RNA binding protein also possesses aconitase activity. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10109–10113. doi: 10.1073/pnas.88.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. C., Beinert H. The state of cluster SH and S2- of aconitase during cluster interconversions and removal. A convenient preparation of apoenzyme. J Biol Chem. 1988 Jun 15;263(17):8194–8198. [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983 Sep 25;258(18):11098–11105. [PubMed] [Google Scholar]
- Kennedy M. C., Kent T. A., Emptage M., Merkle H., Beinert H., Münck E. Evidence for the formation of a linear [3Fe-4S] cluster in partially unfolded aconitase. J Biol Chem. 1984 Dec 10;259(23):14463–14471. [PubMed] [Google Scholar]
- Klausner R. D., Harford J. B. cis-trans models for post-transcriptional gene regulation. Science. 1989 Nov 17;246(4932):870–872. doi: 10.1126/science.2683086. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauble H., Kennedy M. C., Beinert H., Stout C. D. Crystal structures of aconitase with isocitrate and nitroisocitrate bound. Biochemistry. 1992 Mar 17;31(10):2735–2748. doi: 10.1021/bi00125a014. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Pollitt S., Zalkin H. Role of primary structure and disulfide bond formation in beta-lactamase secretion. J Bacteriol. 1983 Jan;153(1):27–32. doi: 10.1128/jb.153.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromou C., Artymiuk P. J., Guest J. R. The aconitase of Escherichia coli. Nucleotide sequence of the aconitase gene and amino acid sequence similarity with mitochondrial aconitases, the iron-responsive-element-binding protein and isopropylmalate isomerases. Eur J Biochem. 1992 Mar 1;204(2):599–609. doi: 10.1111/j.1432-1033.1992.tb16673.x. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Tang C. K., Kaptain S., Burgess W. H., Haile D. J., Samaniego F., McBride O. W., Harford J. B., Klausner R. D. Cloning of the cDNA encoding an RNA regulatory protein--the human iron-responsive element-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7958–7962. doi: 10.1073/pnas.87.20.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydén L., Ofverstedt L. G., Beinert H., Emptage M. H., Kennedy M. C. Molecular weight of beef heart aconitase and stoichiometry of the components of its iron-sulfur cluster. J Biol Chem. 1984 Mar 10;259(5):3141–3144. [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Walden W. E., Patino M. M., Gaffield L. Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13765–13769. [PubMed] [Google Scholar]
- Yu Y., Radisky E., Leibold E. A. The iron-responsive element binding protein. Purification, cloning, and regulation in rat liver. J Biol Chem. 1992 Sep 15;267(26):19005–19010. [PubMed] [Google Scholar]
- Zheng L., Andrews P. C., Hermodson M. A., Dixon J. E., Zalkin H. Cloning and structural characterization of porcine heart aconitase. J Biol Chem. 1990 Feb 15;265(5):2814–2821. [PubMed] [Google Scholar]
- Zheng L., Kennedy M. C., Beinert H., Zalkin H. Mutational analysis of active site residues in pig heart aconitase. J Biol Chem. 1992 Apr 15;267(11):7895–7903. [PubMed] [Google Scholar]