Abstract
This study investigated the diagnostic value of soluble urokinase plasminogen activator receptor (suPAR) and serum lactate in elderly patients with sepsis and evaluated their capacity to predict mortality and their correlation to Sequential Organ Failure Assessment (SOFA) score. The study included 80 participants, divided into two groups: 40 cases (mean age, 68.9 ± 5.9) admitted to the intensive care unit and 40 healthy controls (mean age, 67.1 ± 6.2). Elderly patients with sepsis had significantly higher levels of serum suPAR and lactic acid compared to healthy controls. Receiver operating characteristic (ROC) curve analysis showed that suPAR (cutoff value, ≥4.37 ng/ml) has higher area under the curve (AUC) than lactic acid (cutoff value, ≥1.95 mmol/l) for diagnosing sepsis. Serum lactate has superior prognostic value compared to suPAR with AUC of 0.82 (cutoff value, 2.2 mmol/l) and 0.72 (cutoff value, 6.3 ng/ml), respectively. The diagnostic power of combined usage of suPAR and lactate serum concentrations showed AUC of 0.988 (95% confidence interval 0.934 to 1.0). The combination of both biomarkers either together or with SOFA score may serve as a useful guide to patients who need more intensive resuscitation.
Keywords: sepsis, prognosis, diagnosis, geriatrics, biomarkers, bacterial
Introduction
Bacterial infections and sepsis are commonly encountered problems in critically ill patients, serving both as a cause of admission to intensive care units (ICUs) and healthcare-associated infection following admission [1]. Elderly patients are particularly susceptible to infections and sepsis due to multiple factors including concomitant medical comorbidities, malnutrition, and instrumentation as well as immunosenescence, a decline in immune function that is characterized by chronic, low-grade, systemic inflammation, and impaired responses to immune challenge [2]. Sepsis is one of the commonest causes of death in critically ill patients, especially elderly. Thus, its early diagnosis is extremely important for the institution of timely and specific treatment [3, 4].
Sepsis is defined as the presence (probable or documented) of infection together with systemic manifestations of infection [5]. Nevertheless, the diagnosis of sepsis remains a challenge, considering its complex pathophysiology and heterogeneous symptomatology. Moreover, the clinical presentation of sepsis is often atypical, complicating and potentially delaying diagnosis and treatment, leading to poor outcomes [6]. Despite its prevalence, no standard diagnostic test has been developed to detect the onset and diagnosis of sepsis [7].
Multiple clinical scores were developed for severity and outcome prediction in critically ill patients. Prominent among these is the Sequential Organ Failure Assessment (SOFA) score, a sepsis mortality risk algorithm which includes multiple laboratory and clinical measures, which has shown to be predictive of fatal outcome in the critically ill [8].
Biomarkers, biological molecules that are characteristics of normal or pathogenic processes, can be useful indicators to clinicians. An ideal biomarker for identifying patients that need more intense monitoring and treatment should be both accurate and readily obtainable bedside [9]. A vast range of biomarkers has been proposed in the field of sepsis [10]. C-reactive protein (CRP) is considered the most widely studied and used biomarker in patients with sepsis [11, 12]. Studies of critically ill patients showed that elevated plasma concentrations of CRP were correlated with an increased risk of organ failure and/or death [10]. CRP along with procalcitonin is currently used as sepsis biomarker in many settings [13, 14]. Although there is general agreement on the superior performance of procalcitonin over CRP, the disadvantage of its elevation in absence of bacterial infection as in massive stress, trauma, and surgery, render its use more applicable in medical patients rather than surgical ones [10].
Soluble urokinase plasminogen activator receptor (suPAR) is another proposed sepsis biomarker [15]. The uPAR receptor is expressed on different cell types including neutrophils, lymphocytes, monocytes, macrophages, certain cancer cells, and vascular endothelial cells [16]. uPAR and its ligand, uPA, are participants in numerous immunologic functions including migration, adhesion, angiogenesis, fibrinolysis, and cell proliferation and have been found to promote tissue invasion in malignant diseases [17]. After cleavage from the cell surface, the soluble receptor, suPAR, can be found in the blood and other organic fluids in all individuals [10]. Increased activation of the immune system caused by different types of infections results in increased suPAR concentrations in body fluids [17]. Several studies have indicated that suPAR concentrations may reflect the severity of infection and reported that higher suPAR levels are associated with a worse outcome in a range of noninfectious and infectious diseases [16, 18].
Lactic acid, another biomarker, is not just a byproduct of inadequate blood perfusion but is also considered as a marker of strained cellular metabolism that could happen during stress, critical illness, or increased bacterial load. In addition, elevated levels of lactate may precede clinical evidence of hypoperfusion such as hypotension [19, 20].
This study aimed to investigate the diagnostic value of both suPAR and serum lactate in elderly patients with sepsis and to evaluate their capacity to predict mortality and their correlation to SOFA score.
Methodology
Study design
This prospective observational study was conducted at Ain Shams University Hospitals in Cairo, Egypt, between May, 2013 and February 2014 following approval of the local ethical committee.
Patients and controls
Eighty participants were prospectively included in this study. They were divided into two groups: 40 cases (21 males and 19 females; mean age, 68.93 ± 5.92) admitted to the Geriatric and surgical ICUs and 40 healthy controls (23 males and 17 females; mean age, 67.1 ± 6.2).
Criteria for inclusion in the study were: age over 60 years and patients with suspected or verified underlying infection who met the criteria of sepsis based on the 2001 International Sepsis Definitions Conference criteria [21]. Exclusion criteria were: declining participation by the patient or the next of kin, major trauma or surgical intervention within the last 72 h, and missing data or loss of follow-up to determine patient’s fate. The patients group was further divided into survivors or nonsurvivors, depending on mortality within 30 days after study entry.
Data collection
Data of complete diagnostic workup for each patient was recorded in a case report form (CRF). It included sociodemographics and clinical data (admission condition, clinical diagnosis, comorbidities, source (focus) of infection, duration of hospitalization, and mortality) in addition to results of routine laboratory tests and bacteriological cultures results.
SOFA score was determined upon diagnosing sepsis using measurements recorded in the CRF. The score assesses dysfunction in six different organs (lung, liver, kidney, coagulation, cardiovascular, and central nervous system) using scores ranging from 0 to 24 (from 0 to 4 for each of six organ systems), with higher scores indicating more severe organ dysfunction [22].
Sample collection for CRP, lactic acid, and suPAR
Blood samples were collected by peripheral venipuncture in serum separator tubes, allowed to clot for 30 min, and then centrifuged for 15 min at 1000× g. Serum was separated, aliquoted, and stored at –20 °C until used. Repeated freeze–thaw cycles were avoided.
CRP measurement
Serum CRP concentrations were measured by the turbidimetric latex agglutination method (CRP-Latex, Bio-Systems SA, Barcelona, Spain) with a detection limit of 1.0 mg/l.
suPAR measurement
Serum suPAR concentrations were measured using a commercial enzyme-linked immunosorbent assay (ELISA) (Quantikine Human uPAR Immunoassay, R&D Systems, Minneapolis, USA) following the manufacturer’s instructions. The immunoassay is a 4.5-hour solid-phase ELISA designed to measure human uPAR in cell culture supernates, serum, plasma, and urine. Optical densities at 450 nm with background to subtract at 570 nm were read within 30 min of adding stop solution.
Lactic acid measurement
Serum lactate concentrations were determined using Cobas 6000 fully automated analyzer (Roche Diagnostics).
Statistical analysis
Continuous variables are expressed as mean and standard deviation, median, and interquartile range according to data distribution. Categorical variables are expressed as frequencies and percent. Mann–Whitney test was used to assess the statistical significance of the difference between cases and controls regarding non parametric variables. Spearman’s correlation was used to assess the correlation between variables. The receiver operating characteristic (ROC) curve was used to evaluate the sensitivity and specificity of the proposed biomarkers in diagnosis of cases and in prediction of mortality among cases. Logistic regression was used to combine suPAR and CRP, and suPAR and lactic acid for prediction of mortality. A significance level of p < 0.05 was used in all tests. All statistical procedures were carried out using SPSS version 15 for Windows (SPSS Inc., Chicago, IL, USA).
Ethics statement
This prospective observational study was conducted at Ain Shams University (ASU) Hospitals in Cairo, Egypt, between May, 2013 and February 2014. Informed consents were obtained from all study participants. The work has been approved by ASU Ethics Committee and was carried out in accordance with the ethical guidelines of the Declaration of Helsinki, 1975.
Results
Demographic and clinical characteristics of the studied subjects (n = 80) are summarized in Table 1.
Table 1.
Demographic and clinical characteristics of the patients (n = 40) and controls (n = 40)
| Characteristics | Cases | Controls | p value |
|---|---|---|---|
| Age (years) | 68.93 ± 5.92 | 67.1 ± 6.2 | >0.05 |
| Sex | |||
| Female | 21 (52.5%) | 17 (42.5%) | >0.05 |
| Male | 19 (47.5%) | 23 (57.5%) | |
| Focus of sepsis | |||
| Respiratory tract infection | 31 (77.5%) | ||
| Urinary tract infection | 4 (10%) | ||
| Mixed infections* | 5 (12.5%) | ||
| Patients’ outcome | |||
| Survived | 16 (40%) | ||
| Died | 24 (60%) | ||
| CRP (mg/l) | 154 ± 92.5 | ||
| SOFA score | 6 [4.5–7] | ||
*Mixed infections included 4 patients (10%) with respiratory and urinary tract infection and one patient with urinary tract infections and infected pressure ulcer (2.5%)
Abbreviations: CRP, C-reactive protein; SOFA, Sequential Organ Failure Assessment
Data are presented as mean ± standard deviation or median [interquartile range] for continuous variables and as number (percentage) for categorical variables
Diagnostic value of suPAR and lactic acid
Serum levels of suPAR and lactic acid were highly significant in patients with sepsis compared to healthy controls (p < 0.001) (Fig. 1). The accuracy of suPAR and lactic acid is illustrated in Table 2. ROC curve analysis showed that suPAR has higher area under the curve (AUC) than lactic acid for diagnosing sepsis (Fig. 2). At a cutoff value of ≥4.37 ng/ml, the sensitivity and specificity of suPAR were 97.5% and 90%, respectively, while the sensitivity and specificity of lactic acid were 67.5% and 87.5%, respectively, at a cutoff value of ≥1.95 mmol/l. The diagnostic power of combined usage of suPAR and lactic acid serum concentrations showed AUC of 0.988 (95% confidence interval [CI] 0.934 to 1.0, p < 0.001) (data not shown).
Fig. 1.

Box plots showing serum concentrations of suPAR and lactic acid in patients (n = 40) and controls (n = 40). A: Serum levels of suPAR were highly significant in patients with sepsis (median = 7.75 ng/ml) compared to healthy controls (median = 3 ng/ml) (p < 0.001). B: Serum levels of lactic acid were highly significant in patients with sepsis (median = 2 mmol/l) compared to healthy controls (median = 1.05 mmol/l) (p < 0.001)
Table 2.
Accuracy of suPAR and lactic acid in diagnosing sepsis in elderly patients
| Biomarker | Cutoff value | AUC (95% CI) | Sensitivity | Specificity | PPV | NPV | p value |
|---|---|---|---|---|---|---|---|
| suPAR (mg/dl) | ≥4.37 | 0.99 (0.93 to 1.0) | 97.5% | 90.0% | 90.7% | 97.3% | 0.001* |
| Lactic acid (mmol/l) | ≥1.95 | 0.84 (0.74 to 0.91) | 67.5% | 87.5% | 84.4% | 72.9% | 0.001* |
Abbreviations: AUC, area under ROC (receiver operating characteristic) curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value
*Highly significant difference
Fig. 2.
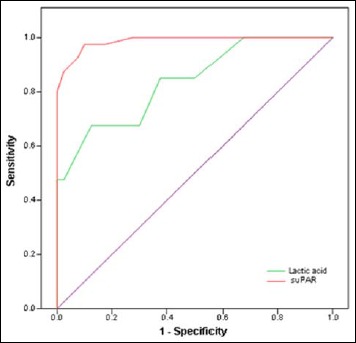
Receiver operating characteristic (ROC) curve analysis of suPAR and lactic acid for diagnosing sepsis in elderly patients. suPAR has higher area under the curve (AUC = 0.99) than lactic acid (AUC = 0.84) for diagnosing sepsis
Correlation between levels of CRP, suPAR, lactic acid, and SOFA scores
As shown in Fig. 3, serum levels of suPAR showed highly significant correlation with serum lactic acid levels (r = 0.414, p = 0.008) in patients with sepsis and a weaker correlation with SOFA scores (r = 0.373, p = 0.018). SOFA scores also correlated with levels of lactic acid (r = 0.403, p = 0.010). On the other hand, serum CRP levels did not correlate with the levels of suPAR, lactic acid, or SOFA scores (data not shown).
Fig. 3.
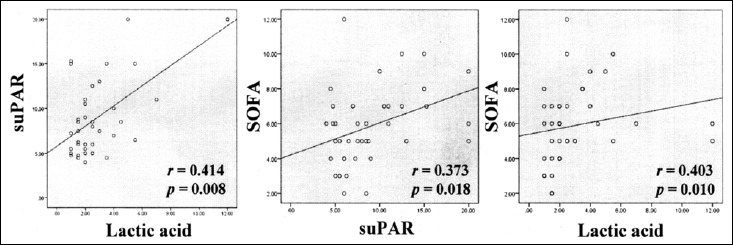
Correlations of serum levels of suPAR, lactic acid, and SOFA score in elderly patients with sepsis (r: Spearman correlation coefficient). Serum levels of suPAR showed highly significant correlation with serum lactic acid levels (r = 0.414, p = 0.008) in patients with sepsis and a weaker correlation with SOFA scores (r = 0.373, p = 0.018). SOFA scores correlated with levels of lactic acid (r = 0.403, p = 0.010)
Prognostic value of CRP, suPAR, and lactic acid
Patients with fatal outcome had significantly higher SOFA score as expected in addition to significantly higher serum levels of lactic acid and suPAR (Fig. 4). The accuracy of the CRP, suPAR, lactic acid, and SOFA score in predicting mortality is summarized in Table 3. Serum lactate has superior prognostic value compared to other biomarkers with an AUC of 0.82 (cutoff value, 2.2 mmol/l; sensitivity, 66.7%; specificity, 81.3%) followed by suPAR with an AUC of 0.72 (cutoff value, 6.3 ng/ml; sensitivity, 79.2%; specificity, 62.5%). The performance of suPAR improved when combined with lactic acid, showing an AUC of 0.852 (95% CI: 0.704 to 0.944) (Table 4).
Fig. 4.
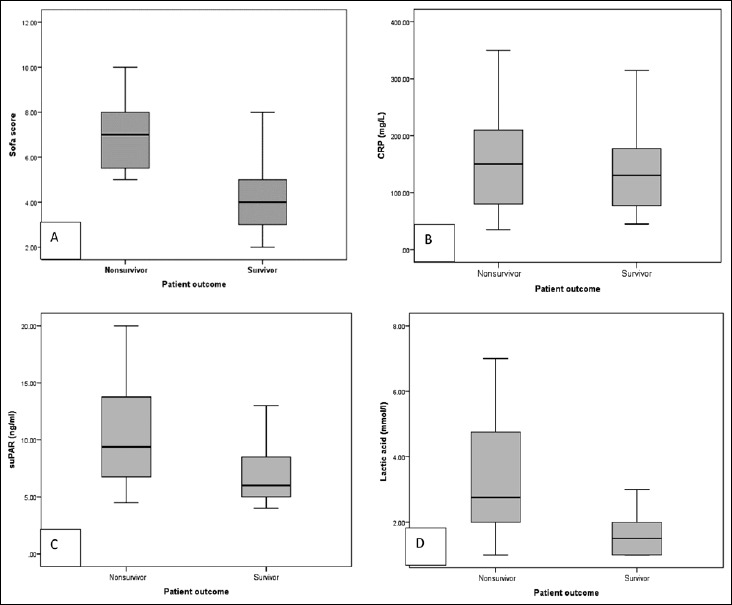
Box plots showing results of SOFA scores and serum concentrations of the studied biomarkers in nonsurvivors (n = 24) and survivors (n = 16). A: Nonsurvivors had significantly higher SOFA scores compared to survivors (p < 0.001). B: There was no statistical significant difference between serum levels of CRP in nonsurvivors (median = 150 mg/l) and survivors (median = 130 mg/l) (p = 0.392). C: There was statistical difference between serum levels of suPAR in nonsurvivors (median = 9.38 ng/ml) and survivors (median = 6 ng/ml) (p = 0.01). D: Serum levels of lactic acid were highly significant in in non survivors (median = 2.75 mmol/l) compared to survivors (median = 1.5 mmol/l) (p < 0.001)
Table 3.
Accuracy of SOFA score, CRP, suPAR, and lactic acid in predicting mortality in elderly patients with sepsis
| Cutoff value | AUC (95% CI) | Sensitivity | Specificity | PPV | NPV | p value | |
|---|---|---|---|---|---|---|---|
| SOFA score | ≥4.5 | 0.88 (0.745 to 0.964) | 100% | 62.5% | 80% | 100% | 0.001** |
| CRP (mg/l) | ≥145 | 0.58 (0.410 to 0.730) | 58.3% | 62.5% | 70% | 50% | 0.372 |
| suPAR (ng/ml) | ≥6.3 | 0.72 (0.560 to 0.853) | 79.2% | 62.5% | 76% | 66.7% | 0.018* |
| Lactic acid (mmol/l) | ≥2.2 | 0.82 (0.667 to 0.923) | 66.7% | 81.3% | 84.2% | 61.9% | 0.001** |
Abbreviations: AUC, area under ROC (receiver operating characteristic) curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value
*Significant difference
**Highly significant difference
Table 4.
Combined ROC curve for suPAR and each of lactic acid, CRP, and SOFA score for prediction of mortality among elderly patient with sepsis
| AUC | Standard error | 95% CI | p value | |
|---|---|---|---|---|
| suPAR + CRP | 0.738 | 0.0782 | 0.575 to 0.864 | 0.02* |
| suPAR + lactic acid | 0.852 | 0.0598 | 0.704 to 0.944 | 0.0002** |
| suPAR + SOFA | 0.902 | 0.048 | 0.766 to 0.973 | 0.0001** |
| SOFA + CRP | 0.883 | 0.053 | 0.742 to 0.963 | 0.0001** |
| Lactic acid + CRP | 0.841 | 0.06 | 0.691 to 0.937 | 0.0001** |
Abbreviations: AUC, area under ROC (receiver operating characteristic) curve; CI, confidence interval
*Significant difference
**Highly significant difference
Discussion
New rapid and accurate approaches for diagnosing sepsis in critically ill patients have been a recent target for scientific research. In this context, many biomarkers have been evaluated to assist either in diagnosing sepsis or in predicting patients’ outcome [23].
Of the many proposed biomarkers, suPAR has gained the interest of many researchers lately. There has been conflicting findings concerning its importance as a potential biomarker for sepsis. The current study showed significantly higher levels of serum suPAR in elderly patients with sepsis compared to healthy controls with a cutoff value of ≥4.37 mg/dl, AUC of 0.99, 97.5% sensitivity, and 90% specificity. The high sensitivity and specificity levels in this study at a relatively low cutoff value could be attributed to the fact that the control group was selected from healthy elders. Our findings support those of previously published researches, in which significantly higher levels of suPAR were found in patients with sepsis compared to non-septic patients [9, 24] and in patients with blood culture-positive bacteremia compared to healthy controls [25], and showed a high predictive capacity for bacteremia in ICU patients [26]. It was also found to be significantly higher in critically ill patients compared to healthy controls [15, 17, 27, 28]. On the other hand, some studies revealed that systematic levels of suPAR have little diagnostic value in critically ill patients with sepsis, SIRS, or bacteremia as shown by Backes and colleagues in their systematic review about usefulness of suPAR as a biological marker in patients with systemic inflammation or infection [29].
Good correlation between high suPAR levels and mortality among patients with sepsis has been illustrated in a number of recently published studies [17, 24, 30]. This agreed with the results of the present work, as we found that serum levels of suPAR were significantly higher in patients with sepsis who had fatal outcomes compared to patients who survived sepsis with an AUC of 0.72 (cutoff value, 6.3 ng/ml; sensitivity, 79.2%; specificity, 62.5%; PPV, 76%). Also, this value agreed best with the results of Uusitalo-Seppälä and colleagues [31], who conducted a cohort study comprised of 539 patients in the emergency department with suspected infection. They found that levels of suPAR were significantly higher in nonsurvivors compared with survivors, that is, at a cut-off level of 6.4 ng/ml, showing comparable sensitivity and specificity rates to ours. Also, the described results of elevated suPAR in sepsis nonsurvivors came in accordance with those reported by a study enrolling 197 patients with sepsis due to a variety of infectious diseases which detected a cutoff value for suPAR of 8 ng/ml, and a study enrolling 180 patients with ventilator-associated pneumonia and sepsis [17, 32]. However, in the later study, a higher cutoff value was detected, where suPAR level greater than 12.9 ng/ml had 80% specificity and 76.1% positive predictive value for prognosis of unfavorable outcome.
Limited data is published about the role of serum lactate per se as a diagnostic or prognostic marker of sepsis especially among the older age groups and whether the same cutoff values as younger age groups can be applied.
In this study, serum lactate was significantly higher in patients with sepsis with a mean value of 3 ± 2.52 mmol/l compared to healthy controls who showed a mean value of 1.2 ± 0.5 mmol/l (p = 0.001). A cutoff value of 1.95 mmol/l was associated with 67.5% sensitivity and 87.5% specificity. Singer et al. reported that at a cut of value of as low as 2 mmol/l showed better specificity than sensitivity, concluding that a normal lactate should not be used to exclude sepsis (even severe sepsis), while an elevated lactate level, especially when greater than 4 mmol/l, is highly specific for sepsis [20].
In a number of other studies and published guidelines, sepsis with lactate level greater than or equal to 4 mmol/l was found to be associated with high mortality and is an indication to initiate treatment protocols and care bundles [4, 33, 34]. Yet, in this study, a cutoff value of 2.2 mmol/l has a sensitivity of 66.7% and specificity of 81% in predicting mortality among elderly patients with sepsis. This relatively low value could be attributed to the older age of patients included in the study who may have a higher risk of bad prognosis even in apparently stable hemodynamics. However, still, our results came in accordance with those of Howell et al. [35] and Mikkelsen et al. [36] who found that hemodynamically stable patients with intermediate serum lactate levels (2–3.9 mmol/l) experienced mortality twice that of the low serum lactate group and with those of Del Portal et al. [37], who also conducted their study on older patients. Our findings, thus, give more strength to the question raised by the later authors of whether the serum lactate threshold used to define severe sepsis needs to be lowered so that these patients may benefit from a more aggressive resuscitation strategy.
In the current study, serum levels of suPAR showed highly significant correlation with serum lactic acid levels (r = 0.414, p = 0.008) in elderly patients with sepsis. Also, the diagnostic power of combined usage of suPAR and lactic acid serum concentrations showed AUC of 0.988. These findings encourage others to focus on this point in upcoming research, as there is no available data on the use of this duet.
Supporting previous findings published by Gustafsson et al. [9], Huttunen et al. [8], Kofoed et al. [18], and Mölkänen et al. [30], our results showed that suPAR levels correlated to SOFA scores in patients with sepsis (r = 0.373, p = 0.018), confirming that suPAR levels are closely linked to disease severity in critical illness. On the other hand, we found no correlation between suPAR levels and the nonspecific inflammatory marker, CRP in sepsis patients, which is consistent with a multicenter study conducted by Wittenhagen et al. to investigate the level of suPAR in patients with Streptococcus pneumoniae bacteremia. They compared suPAR and CRP levels and found no correlation between them (r = 0.004; p = 0.96) [25].
The current study tested the prognostic predictive power of hypothesized duets in the form of combined ROC curves. SOFA score and suPAR showed best results with an AUC of 0.902 followed by the combination of SOFA score with CRP (AUC = 0.883). The later combination, though not the best performance, would set a realistic option for limited resource settings like most of our local health care facilities, considering the more availability and cost effectiveness of CRP.
Limitations of the current study
The study was based on a relatively small sized sample that included cases and healthy controls only. The addition of another group of critically ill patients would have been beneficial to the study through testing the ability of the proposed biomarkers to discriminate between sepsis and systemic inflammatory response syndrome.
Conclusion
This study showed a promising ability of suPAR to serve as a biomarker in diagnosing sepsis and predicting mortality among elderly patients indicated by significantly higher levels in patients compared to controls, and the superior predictive value of lactic acid as a single biomarker in predicting mortality among the same cohort. The study also highlights the usefulness of combination of both biomarkers either together or with SOFA score as a guide to patients who need more intensive resuscitation. Still, more studies on wider scales are needed to elucidate the best performing biomarker and the clinical usefulness of these combinations.
Funding Statement
Funding sources There is no funding to declare.
References
- 1.Kibe S, Adams K, Barlow G: Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother 66(Suppl. 2), 33–40 (2011) [DOI] [PubMed] [Google Scholar]
- 2.Wester AL, Dunlop O, Melby KK, Dahle UR, Wyller TB: Age-related differences in symptoms, diagnosis and prognosis of bacteremia. BMC Infect Dis 13(1), 346 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall JC Vincent J-L Fink MP et al.: Measures, markers, and mediators: toward a staging system for clinical sepsis. A report of the Fifth Toronto Sepsis Roundtable, Toronto, Ontario, Canada, October 25–26, 2000. Crit Care Med 31(5), 1560–1567 (2003) [DOI] [PubMed] [Google Scholar]
- 4.Dellinger RP, Levy MM, Carlet JM, et al. : Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dellinger RP Levy MM Rhodes A et al.: Surviving Sepsis Campaign. Crit Care Med 41(2), 580–637 (2013) [DOI] [PubMed] [Google Scholar]
- 6.Lee S-H, Chan R-C, Wu J-Y, Chen H-W, Chang S-S, Lee C-C: Diagnostic value of procalcitonin for bacterial infection in elderly patients – a systemic review and meta-analysis. Int J Clin Pract 67(12), 1350–1357 (2013) [DOI] [PubMed] [Google Scholar]
- 7.Drewry AM, Hotchkiss RS: Sepsis: Revising definitions of sepsis. Nat Rev Nephrol 11(6), 326–328 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huttunen R Syrjänen J Vuento R et al.: Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: a prospective cohort study. J Intern Med 270(1), 32–40 (2011) [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson A, Ljunggren L, Bodelsson M, Berkestedt I: The prognostic value of suPAR compared to other inflammatory markers in patients with severe sepsis. Biomark Insights 7, 39–44 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinhart K, Bauer M, Riedemann NC, Hartog CS: New approaches to sepsis: molecular diagnostics and biomarkers. Clin Microbiol Rev 25(4), 609–634 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kofoed K, Andersen O, Kronborg G, et al. : Use of plasma C-reactive protein, procalcitonin, neutrophils, macrophage migration inhibitory factor, soluble urokinase-type plasminogen activator receptor, and soluble triggering receptor expressed on myeloid cells-1 in combination to diagnose infections. Crit Care 11(2), R38 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent J-L, Donadello K, Schmit X: Biomarkers in the critically ill patient: C-reactive protein. Crit Care Clin 27(2), 241–251 (2011) [DOI] [PubMed] [Google Scholar]
- 13.Carrigan SD, Scott G, Tabrizian M: Toward resolving the challenges of sepsis diagnosis. Clin Chem 50(8), 1301–1314 (2004) [DOI] [PubMed] [Google Scholar]
- 14.Meisner M: Biomarkers of sepsis: clinically useful? Curr Opin Crit Care 11, 473–480 (2005) [DOI] [PubMed] [Google Scholar]
- 15.Mizukami IF, Faulkner NE, Gyetko MR, Sitrin RG, Todd RF: Enzyme-linked immunoabsorbent assay detection of a soluble form of urokinase plasminogen activator receptor in vivo. Blood 86(1), 203–211 (1995) [PubMed] [Google Scholar]
- 16.Donadello K, Scolletta S, Covajes C, Vincent J-L: suPAR as a prognostic biomarker in sepsis. BMC Med 10(1), 2 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch A, Voigt S, Kruschinski C, et al. : Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care 15(1), R63 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kofoed K, Eugen-Olsen J, Petersen J, Larsen K, Andersen O: Predicting mortality in patients with systemic inflammatory response syndrome: an evaluation of two prognostic models, two soluble receptors, and a macrophage migration inhibitory factor. Eur J Clin Microbiol Infect Dis 27(5), 375–383 (2008) [DOI] [PubMed] [Google Scholar]
- 19.Blomkalns AL: Lactate – a marker for sepsis and trauma. EMCREG Int 43–49 (2007) [Google Scholar]
- 20.Singer AJ Taylor M Domingo A et al.: Diagnostic characteristics of a clinical screening tool in combination with measuring bedside lactate level in emergency department patients with suspected sepsis. Acad Emerg Med 21(8), 853–857 (2014) [DOI] [PubMed] [Google Scholar]
- 21.Levy MM Fink MP Marshall JC et al.: 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29(4), 530–538 (2003) [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL de Mendonça A Cantraine F et al.: Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26(11), 1793–1800 (1998) [DOI] [PubMed] [Google Scholar]
- 23.Pierrakos C, Vincent J-L: Sepsis biomarkers: a review. Crit Care 14(1), R15 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yilmaz G, Köksal I, Karahan SC, Mentese A: The diagnostic and prognostic significance of soluble urokinase plasminogen activator receptor in systemic inflammatory response syndrome. Clin Biochem 44(14–15), 1227–1230 (2011) [DOI] [PubMed] [Google Scholar]
- 25.Wittenhagen P Kronborg G Weis N et al.: The plasma level of soluble urokinase receptor is elevated in patients with Streptococcus pneumoniae bacteraemia and predicts mortality. Clin Microbiol Infect 10(5), 409–415 (2004) [DOI] [PubMed] [Google Scholar]
- 26.Georgescu A-M Szederjesi J Voidăzan S et al.: Soluble urokinase-type plasminogen activator receptor (suPAR) – a possible biomarker for bacteremia in sepsis/Forma solubilă a receptorului pentru activatorul de plasminogen de tip urokinază (suPAR) – un biomarker posibil pentru bacteriemie în sepsis. Rom Rev Lab Med 23(1), 59–73 (2015) [Google Scholar]
- 27.Florquin S van den Berg JG Olszyna DP et al.: Release of urokinase plasminogen activator receptor during urosepsis and endotoxemia. Kidney Int 59(6), 2054–2061 (2001) [DOI] [PubMed] [Google Scholar]
- 28.Kofoed K, Schneider UV, Scheel T, Andersen O, Eugen-Olsen J: Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem 52(7), 1284–93 (2006) [DOI] [PubMed] [Google Scholar]
- 29.Backes Y Van Der Sluijs KF Mackie DP et al.: Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: A systematic review. Intensive Care Med 38, 1418–1428 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mölkänen T, Ruotsalainen E, Thorball CW, Järvinen A: Elevated soluble urokinase plasminogen activator receptor (suPAR) predicts mortality in Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 30(11), 1417–1424 (2011) [DOI] [PubMed] [Google Scholar]
- 31.Uusitalo-Seppälä R Huttunen R Tarkka M et al.: Soluble urokinase-type plasminogen activator receptor in patients with suspected infection in the emergency room: a prospective cohort study. J Intern Med 272(3), 247–256 (2012) [DOI] [PubMed] [Google Scholar]
- 32.Savva A Raftogiannis M Baziaka F et al.: Soluble urokinase plasminogen activator receptor (suPAR) for assessment of disease severity in ventilator-associated pneumonia and sepsis. J Infect 63(5), 344–350 (2011) [DOI] [PubMed] [Google Scholar]
- 33.Rivers E Nguyen B Havstad S et al.: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 345(19), 1368–1377 (2001) [DOI] [PubMed] [Google Scholar]
- 34.Donnino MW, Nguyen B, Jacobsen G, Tomlanovich M, Rivers E: Cryptic Septic Shock: A Sub-analysis of Early, Goal-Directed Therapy. Chest J 124(4_MeetingAbstracts), 90S–b–90S (2003) [Google Scholar]
- 35.Howell MD, Donnino M, Clardy P, Talmor D, Shapiro NI: Occult hypoperfusion and mortality in patients with suspected infection. Intensive Care Med 33(11), 1892–1899 (2007) [DOI] [PubMed] [Google Scholar]
- 36.Mikkelsen ME Miltiades AN Gaieski DF et al.: Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 37(5), 1670–677 (2009) [DOI] [PubMed] [Google Scholar]
- 37.Del Portal D a., Shofer F, Mikkelsen ME, et al. : Emergency department lactate is associated with mortality in older adults admitted with and without infections. Acad Emerg Med 17(3), 260–268 (2010) [DOI] [PubMed] [Google Scholar]


