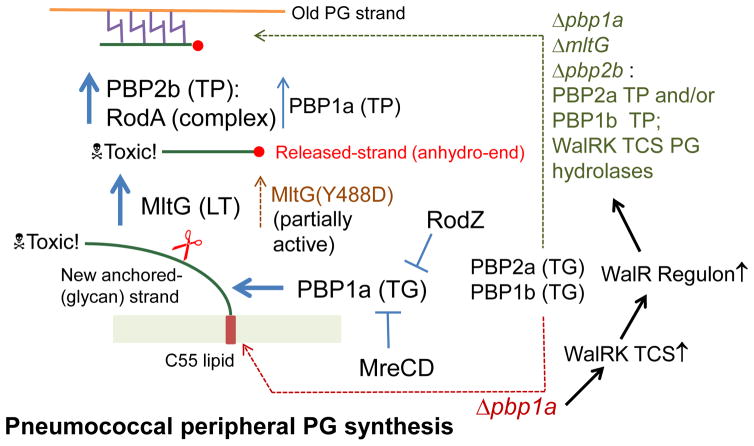
Fig. 12.
Model for the steps in peripheral PG synthesis of Streptococcus pneumoniae in wild-type and Δpbp1a mutant strains. The model proposes that new anchored-glycan PG strand is synthesized primarily by PBP1a (TG) activity, that un-crosslinked strands with anhydro ends are released by the MltG endo-LT, and that released strands are primarily crosslinked into growing peripheral PG by a PBP2b TP activity, which depends on a PBP2b:RodA interaction. Accumulation of the new anchored-glycan strands and released-strands with an anhydro end are assumed to be toxic in mutants. In this model, MreCD and RodZ control PBP1a activity and/or localization, although they may also regulate MltG activity and/or localization (see Discussion). In Δpbp1a mutants, TG activity is provided by other Class A PBPs and MltG still cleaves anchored-glycan strands, along with PG hydrolases induced in amount by activation of the WalRK two-component regulatory system. In a Δpbp1a ΔmltG Δpbp2b mutant an alternative pathway is used that replaces MltG with WalRK-induced PG hydrolases. Evidence is presented that MltG(Y488D) has partial endo-LT activity, and the mltG(Y488D) mutation also induces WalRK TCS regulon expression. The model can account for the phenotypes of mutants presented in this paper. See Discussion for additional details.