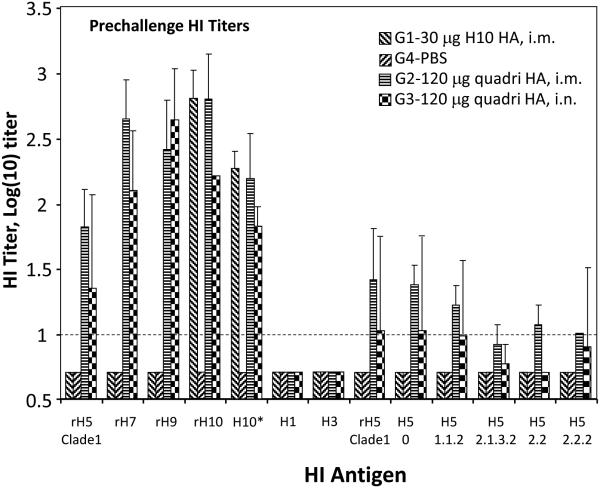
Fig. 4.
Summary of HI antibody titer analysis of the pre-challenge sera from vaccinated ferrets. The HI titers are shown on the Y-axis, while HI antigens used in HI assays are shown on X-axis. The HI antigens were the following: rH5 derived from A/Viet Nam/1203/2004 (Clade 1 H5N1); rH9 from A/Hong Kong/33982/2009 (H9N2); rH7 from A/Anhui/1/2013 (H7N9); and rH10 from A/Jiangxi-DongHu/346/2013 (H10N8). BPL-inactivated viruses included H10* A/teal/Egypt/12908-NAMRU3/2005 (H10N1), H1 A/California/07/2009 NYMC X-197A (H1N1), H3 A/Switzerland/9715293/2013 (H3N2) , as well as several H5N1 viruses, A/Hong Kong/156/1997 (clade 0), A/Cambodia/X012331/2013 (clade 1.1.2), A/Indonesia/05/2005 (clade 2.1.3.2), A/bar-headed goose/Qinghai/1A/2005:PR8, (clade 2.2), and A/Bangladesh/3233/2011 (clade 2.2.2). Dashed horizontal line indicates 1:10 detection limit.