Figure 1.
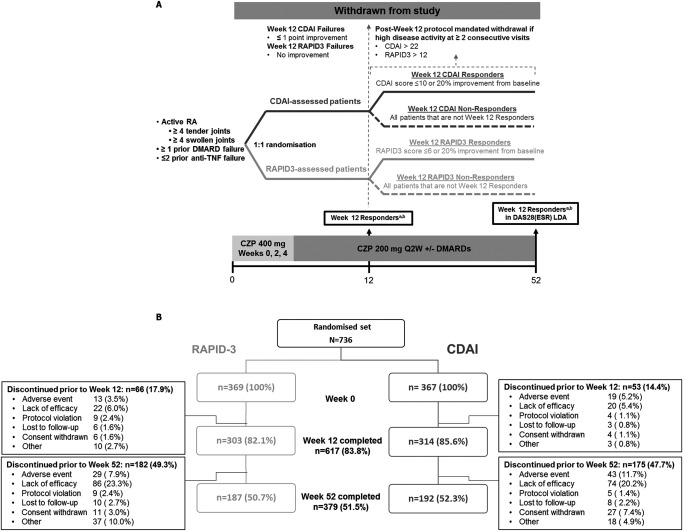
Study design (A) and disposition of the study patients (B). aResponders according to the Clinical Disease Activity Index (CDAI) were defined as patients with a CDAI score of ≤10 or a 20% improvement over baseline. bResponders according to the Routine Assessment of Patient Index Data 3 (RAPID‐3) were defined as patients with a RAPID‐3 score of ≤6 or a 20% improvement over baseline. RA = rheumatoid arthritis; DMARD = disease‐modifying antirheumatic drug; TNF = tumor necrosis factor; DAS28‐ESR = Disease Activity Score in 28 joints using the erythrocyte sedimentation rate; LDA = low disease activity; CZP = certolizumab pegol; Q2W = every 2 weeks.
