Figure 1.
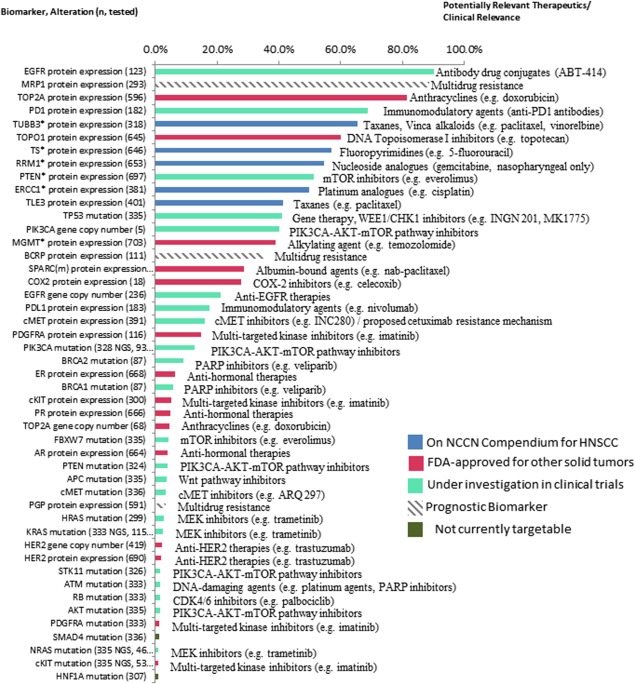
Biomarker alterations and associated clinical relevance, listed in order of frequency observed. Biomarkers are followed by alteration observed (eg, protein expression, mutation, amplification) and n, number of patients assayed. All frequencies are provided in terms of protein overexpression (immunohistochemical [IHC]), increased gene copy number/amplification (in situ hybridization [ISH]), or mutated (next‐generation sequencing [NGS]/Sanger). Biomarkers with * indicate frequency of low or lack of protein expression (IHC), which associates with benefit to associated therapy. Blue bars indicate therapy is On‐NCCN (National Comprehensive Cancer Network) Compendium for head and neck squamous cell carcinoma (HNSCC), red bars indicate therapy is Food and Drug Administration‐approved for other solid tumors, green bars indicate therapy is under investigation in clinical trials, gray hashed bar indicates a prognostic marker, and dark green bars indicate therapy is not currently targetable.
