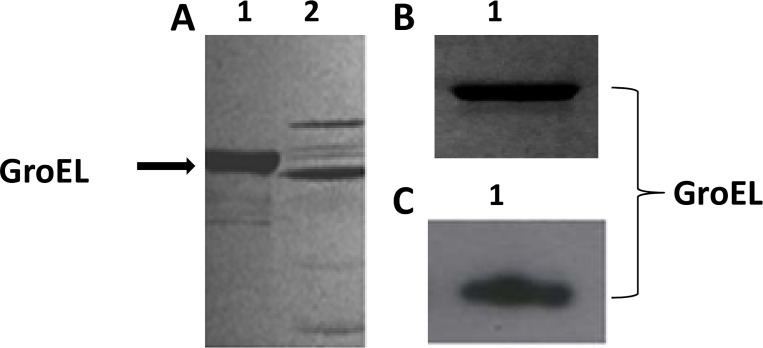
Fig 1. Purified GroEL protein confirmed by western blot analysis.
(A) Purification of AaGroEL by ATP affinity chromatography. A cell extract of Aggregatibacter actinomycetemcomitans was loaded onto an adenosine 5’-triphosphate (ATP) agarose gel and fractions were collected with a 5 mM ATP solution. The ATP fractions were loaded onto an 8% SDS-PAGE gel. Lane 1, recombinant AaGroEL and Lane 2, ATP fraction. (B) Purification of AaGroEL by electroelution. The bands corresponding to the 64-kDa AaGroEL protein were excised from the SDS gel, and the slices were destained and electroeluted for 4 h. Purified AaGroEL was analyzed by SDS-PAGE, and the band was stained with Coomassie brilliant blue R-250. Lane 1, recombinant AaGroEL (200 ng) as a control [16] and Lane 2, electroeluted, purified AaGroEL (200 ng). (C) Native endogenous purified AaGroEL protein was confirmed with western blot analysis using an anti-E. coli GroEL antibody. Lane 1, rAaGroEL (200 ng) and Lane 2, endogenous AaGroEL (200 ng).