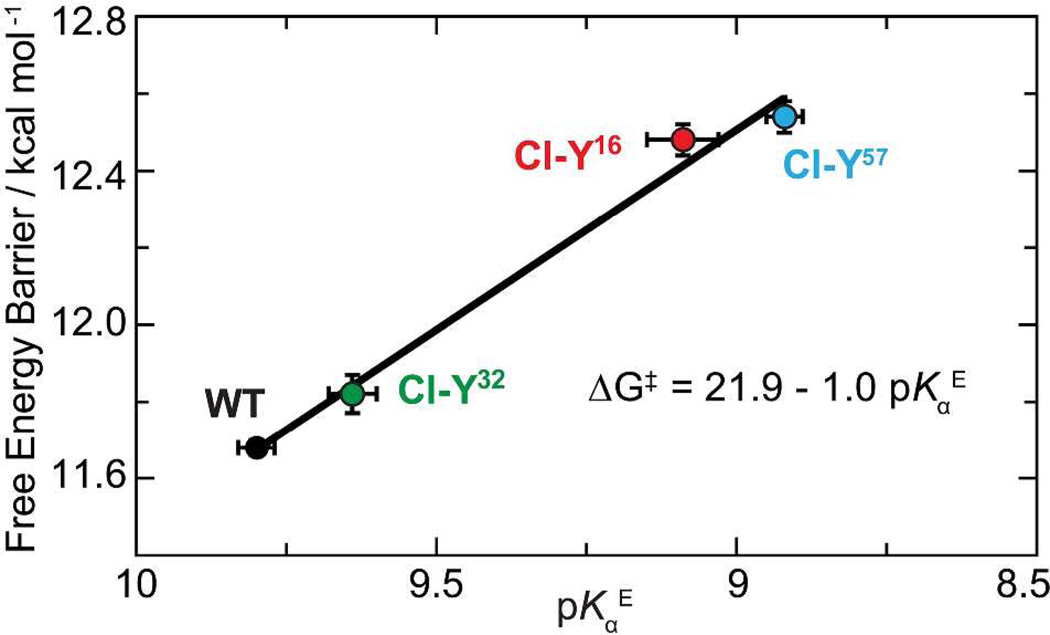
Figure 6. Linear correlation between the proton affinity of KSI’s active site and the catalytic proficiency.
The catalytic proficiency improves linearly as the proton affinity of the enzyme (pKαE) approaches that of the intermediate (pKaI =10) (R2 = 0.99). The extra stabilization energy for barrier reduction is presumably due to the increased H-bond strength between the enzyme and the intermediate, which is estimated to be 6.3 ± 0.2 kcal/mol.