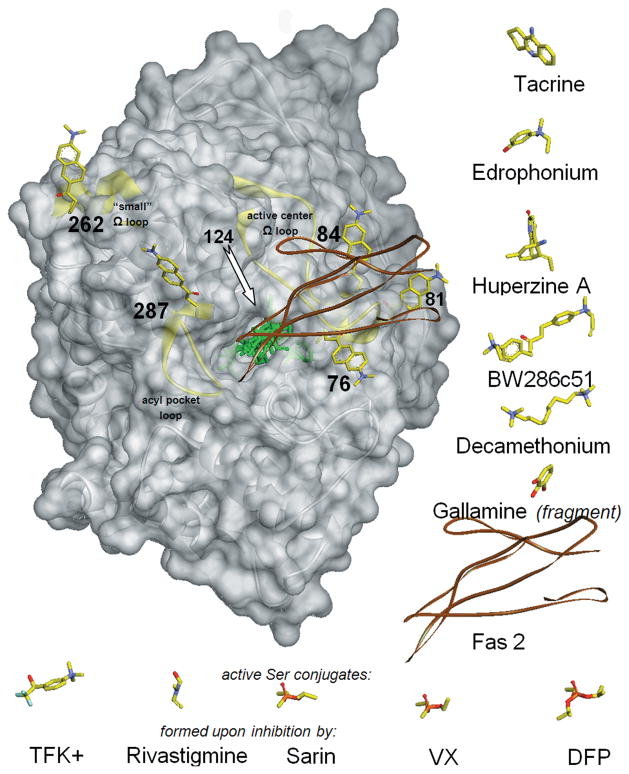
Figure 2.
Structure of mouse AChE (gray transparent Connolly surface and ribbon) with covalently bound fluorescent acrylodan (represented by yellow sticks, docked to AChE manually) shown in each of six individually labeled positions (76, 81, 84,124, 262, and 287). In the centrally located active-center gorge, seven reversible ligands and five covalent conjugates are superimposed (represented by green sticks or brown ribbon for peptide Fas2) in positions resulting from overlays of corresponding ligand–AChE complexes or conjugates that include human, mouse, and Torpedo AChEs. To improve clarity, ligands are also shown next to the AChE molecule, in orientations identical or similar to the ones bound to AChE. Reversible ligands are shown to the right of AChE and covalent conjugates under AChE. Location of the acrylodan bound to position 124 is indicated by the arrow, but the attached acrylodan molecule is not shown, since it overlaps with most of the ligands bound in the center of the AChE gorge. Three structurally independent AChE surface loops (active center Ω loop, acyl pocket loop, and “small” ω loop) are highlighted as yellow ribbons.