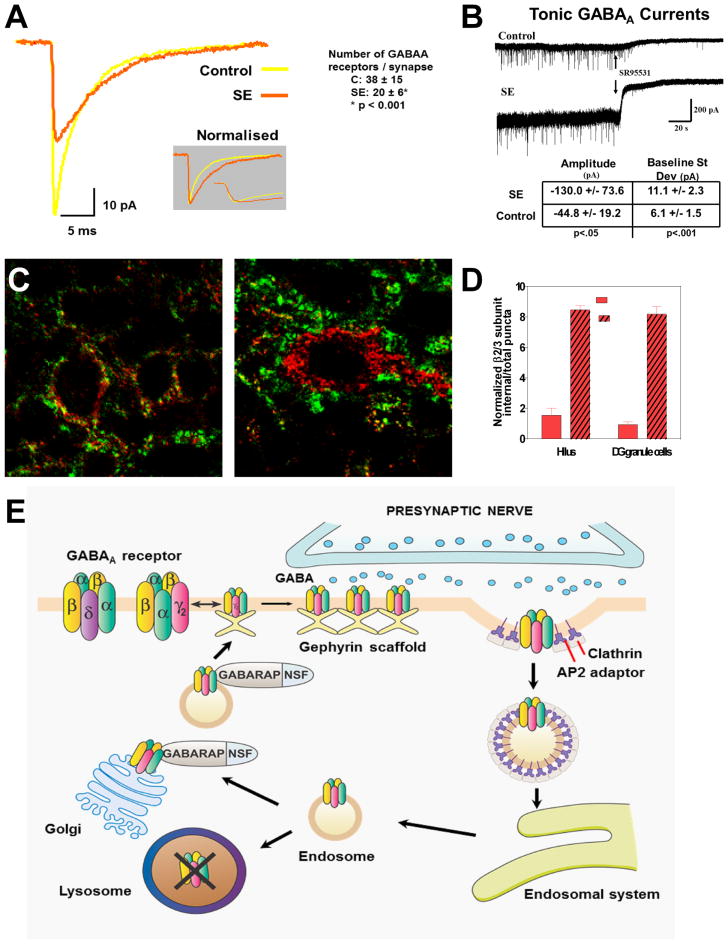
Figure 1.
Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. (A) In hippocampal slices prepared from rats in lithium–pilocarpine–induced status epilepticus (SE) for 1 h, γ-aminobutyric acid (GABA)A miniature inhibitory postsynaptic currents (IPSCs) recorded from granule cells display reduced amplitude that can be attributed primarily to a loss of synaptic receptors (reduced number of GABAA receptors from 38 ± 15 to 20 ± 6 per granule cell synapse). (B) Tonic currents generated by extrasynaptic GABAA receptors are increased in slices from rats in SE, reflecting (at least in part) increased extracellular GABA concentration during SE. (C) Subcellular distribution of β2–3 subunits of GABAA receptors after SE. In control granule cells (left) the β2–3 subunits of GABAA receptors (red) localize to the vicinity of the presynaptic marker synaptophysin (green), whereas after 1 h of SE induced by lithium and pilocarpine (right), many have moved to the cell interior. (D) The graph shows an increase in β2–3 subunit internalization following SE in the hilus and in the dendate gyrus granule cell layer. (E) Model of our hypothesis of GABAA receptor trafficking during the transition of single seizures to status epilepticus. After repeated seizures, the synaptic membrane of GABAA receptors forms clathrin-coated pits, which internalize as clathrin-coated vesicles, inactivating the receptors because they are no longer within reach of the neurotransmitter. These vesicles develop into endosomes, which can deliver the receptors to lysosomes, where they are destroyed, or to the Golgi apparatus, from where they are recycled to the membrane.