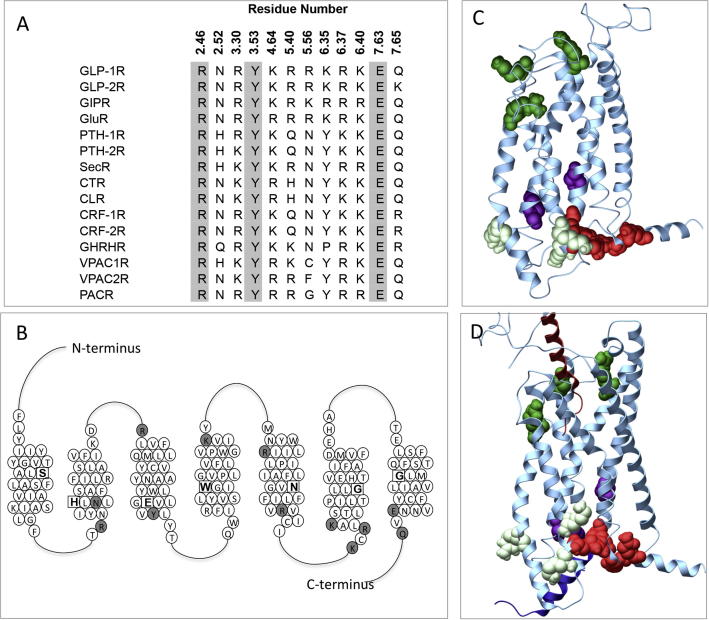
Fig. 1.
Conservation and location of polar residues mutated in this study. (A) Conservation of polar residues mutated in this study across the human Class B GPCRs (the secretin-like subclass). Residues absolutely conserved are highlighted in grey. These residues shown are conserved as polar (with the exception of 5.56 and 6.35 where one receptor subtype is not) across all mammalian species of receptor cloned to date. GLP-1R; glucagon-like peptide-1 receptor, GLP-2R; GLP-2 receptor, GIP, gastric inhibitory polypeptide receptor; GluR, glucagon receptor; PTH-1R, parathyroid hormone receptor 1; PTH-2R, PTH receptor 2; SecR, secretin receptor; CTR, calcitonin receptor; CLR, calcitonin-like receptor; CRF1, corticotropin-releasing factor receptor 1; CRF2, corticotropin-releasing factor receptor 2; GHRHR, GH-releasing hormone receptor; VPAC1R, vasoactive intestinal polypeptide type-1 receptor; VPAC2R, vasoactive intestinal polypeptide type-2 receptor, PACR, pituitary adenylate cyclase activating polypeptide 1 receptor. (B) Schematic representation of the TM domain of the human GLP-1R. The most conserved residue in each helix is highlighted as a square with a bold letter and represent residue 0.50 for that helix. Residues mutated in the present study are shown in grey. (C) Three-dimensional molecular homology model of the inactive TM bundle of the GLP-1R. (D) Three-dimensional molecular model of the TM bundle of the active full length model of the GLP-1R. The bound GLP-1 peptide is shown dipping into the bundle (dark red helix) and the Gαs peptide fragment bound at the intracellular face is shown in dark blue. In (C) and (D), side chains mutated in this study are highlighted in space fill with dark green indicating positively charged residues located towards the extracellular face of the bundle and interact with ECL2; pale green, positively charged residues located towards the intracellular face that may interact with lipid headgroups; red, residues in TMs 2, 6 and 7 that form a hydrogen bond network in the apo receptor; purple, residues in TMs 2 and 3 that stabilise interactions with TM4. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)