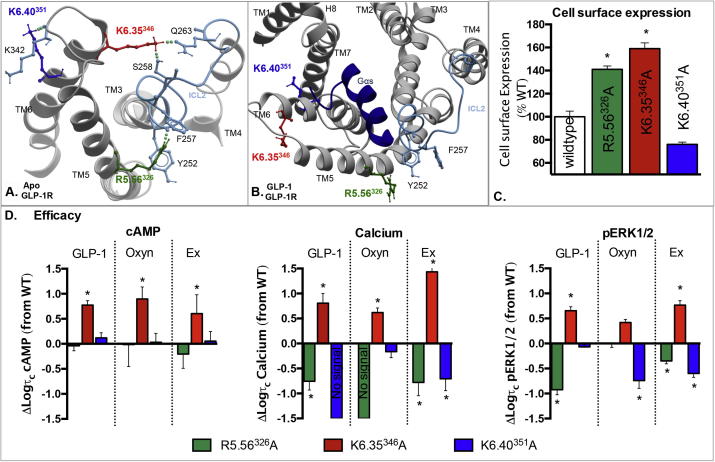
Fig. 8.
Mutation of positively charged residues predicted to interact with ICL2 and/or the lipid bilayer alters cell surface expression and receptor signalling in a pathway dependent manner. (A) TMs 3, 4, 5 and 6 of the apo GLP-1R TM bundle as viewed from the cytoplasmic face, highlighting interactions between charged residues R5.56326 and K6.35346 with residues in ICL2. K6.40351 is also shown where it points away from the bundle, interacting with the backbone of ICL3 and potentially interacting with lipid head groups. (B) The activated GLP-1R TM bundle as viewed from the intracellular face with a Gαs peptide fragment docked at the cytoplasmic face. The lipid facing location of R5.56326, K6.35346 and K6.40351 are highlighted. Of particular note, interactions of K6.35346 with ICL2 are broken to accommodate opening up of the TM bundle and G protein interaction. R5.56326 interactions with the backbone of ICL2 are also broken although R5.56326 maintains within H bond proximity to Y252. (C) Cell surface expression of mutations R5.56326A, K6.35346A and K6.40351A relative to the wildtype receptor as assessed by antibody binding to the N-terminal c-myc epitope tag. (D) Differences in the coupling efficiency (log τc) of GLP-1, exendin-4 and oxyntomodulin to three signalling pathways (cAMP production (left), iCa2+ mobilisation (middle), and pERK1/2 (right)) for R5.56326A, K6.35346A and K6.40351A compared to the wildtype receptor. These log τc were calculated from concentration–response curves presented in Fig. 2, Fig. 3, Fig. 4, and corrected for cell surface expression as measured by antibody labelling recorded in Table 1. Statistical significance of changes in cell surface expression or coupling efficacy in comparison with wildtype were determined by one-way analysis of variance and Dunnett’s post test, and values are indicated with an asterisk (∗, p < 0.05). All values are ± S.E.M of four to six independent experiments, conducted in duplicate.