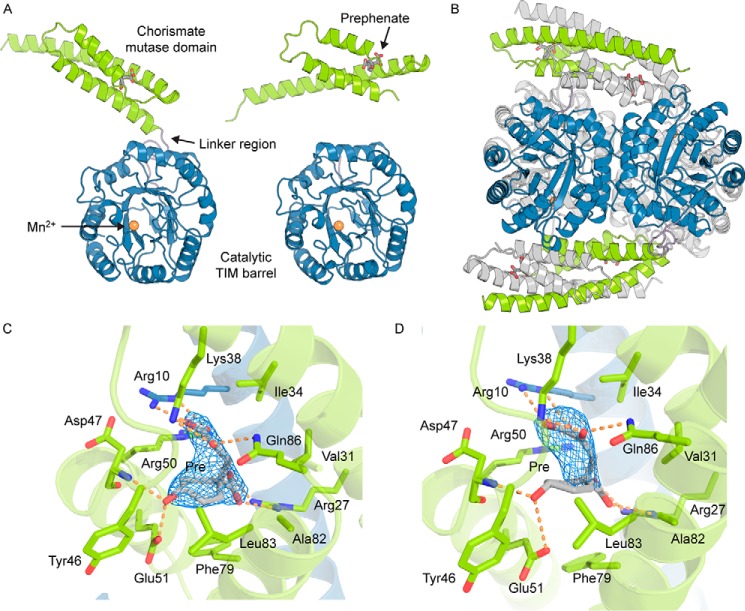
FIGURE 6.
Crystal structure of GspDAH7PS. A, two chains of the asymmetric unit, chain A (left) and chain B (right). The catalytic TIM barrel is shown in blue, the CM domain in green, and the linker region in purple. Allosteric inhibitor prephenate (Pre) is represented with gray sticks, and the active site is identified with the metal ion, represented with an orange sphere. B, tetrameric assembly with the components of one asymmetric unit colored (TIM barrel is shown in blue, the CM domain in green, and the linker region in purple) and the symmetry-related dimer is colored gray. C and D, binding sites of prephenate in the two unique subunits of the asymmetric unit. Residues important for the binding of prephenate and the catalytic activity of the CM domain are shown with sticks. One CM domain is shown in green, and the adjacent chain forming the catalytically active CM is shown in blue. Prephenate is represented with gray sticks. The σA-weighted Fo − Fc omit maps for prephenate are represented with a blue mesh and were generated by deleting the prephenate molecules from the n-1 round of refinement. Electron density is contoured at 3σ (0.1457e/Å3).