Abstract
ATP-sensitive potassium (KATP) channels play a key role in mediating glucose-stimulated insulin secretion by coupling metabolic signals to β-cell membrane potential. Loss of KATP channel function due to mutations in ABCC8 or KCNJ11, genes encoding the sulfonylurea receptor 1 (SUR1) or the inwardly rectifying potassium channel Kir6.2, respectively, results in congenital hyperinsulinism. Many SUR1 mutations prevent trafficking of channel proteins from the endoplasmic reticulum to the cell surface. Channel inhibitors, including sulfonylureas and carbamazepine, have been shown to correct channel trafficking defects. In the present study, we identified 13 novel SUR1 mutations that cause channel trafficking defects, the majority of which are amenable to pharmacological rescue by glibenclamide and carbamazepine. By contrast, none of the mutant channels were rescued by KATP channel openers. Cross-linking experiments showed that KATP channel inhibitors promoted interactions between the N terminus of Kir6.2 and SUR1, whereas channel openers did not, suggesting the inhibitors enhance intersubunit interactions to overcome channel biogenesis and trafficking defects. Functional studies of rescued mutant channels indicate that most mutants rescued to the cell surface exhibited WT-like sensitivity to ATP, MgADP, and diazoxide. In intact cells, recovery of channel function upon trafficking rescue by reversible sulfonylureas or carbamazepine was facilitated by the KATP channel opener diazoxide. Our study expands the list of KATP channel trafficking mutations whose function can be recovered by pharmacological ligands and provides further insight into the structural mechanism by which channel inhibitors correct channel biogenesis and trafficking defects.
Keywords: ABC transporter, ATP-binding cassette transporter subfamily C member 8 (ABCC8), chaperone, gating, hypoglycemia, insulin secretion, intracellular trafficking, potassium channel, protein misfolding, protein processing
Introduction
Protein function relies on the proper folding, assembly, and trafficking to specific cellular compartments. In the case of plasma membrane proteins, such as ion channels and receptors, they must pass quality surveillance in the endoplasmic reticulum (ER)3 to enter the secretory pathway and ultimately reach the cell surface. Numerous diseases arise due to mutations that disrupt protein folding, assembly, and subsequent trafficking to the cell surface (1), hereafter referred to as trafficking mutations. Small molecules termed pharmacological chaperones hold promise as a means of therapy for such diseases by interacting with mutant proteins and correcting their folding and trafficking defects (2–4).
Congenital hyperinsulinism (HI) is a rare, life-threatening disease characterized by persistent insulin secretion despite extreme hypoglycemia (5). The most common cause of HI is loss-of-function mutations in the ABCC8 or KCNJ11 genes encoding the sulfonylurea receptor 1 (SUR1) and inwardly rectifying potassium channel Kir6.2 proteins, respectively (5–7). SUR1 and Kir6.2 form the pancreatic subtype of the ATP-sensitive K+ (KATP) channel, which plays a key role in glucose-stimulated insulin secretion by coupling glucose metabolism to β-cell membrane excitability (7–9). SUR1 or Kir6.2 mutations identified in patients with HI have been shown to disrupt channel gating and/or biogenesis and trafficking (10, 11). Whereas a small number (<10%) of affected individuals are successfully treated with the KATP channel opener diazoxide, those with the more severe form due to loss of channel surface expression are diazoxide-unresponsive and often require partial or total pancreatectomy to avoid severe consequences of hypoglycemia (5). Restoring channel expression with pharmacological chaperones is therefore an attractive alternative therapy.
HI-causing KATP channel trafficking mutations are found throughout both SUR1 and Kir6.2, but most of them are in the larger SUR1 subunit. SUR1 is a member of the ATP-binding cassette (ABC) transporter superfamily (10, 11). In addition to the ABC core domain found in each member (TMD1/NBD1/TMD2/NBD2; see Fig. 1A), SUR1 contains an N-terminal transmembrane domain TMD0 and a long cytoplasmic loop L0, which connects TMD0 to TMD1 (12). We have previously found that sulfonylureas (SUs); KATP channel inhibitors, such as glibenclamide (GBC); and tolbutamide could act as pharmacological chaperones to correct KATP channel trafficking defects (13–16). More recently, we identified a novel KATP channel inhibitor carbamazepine (CBZ), which also rescues trafficking-impaired KATP channels to the cell surface (17, 18). Remarkably, the SUR1 trafficking mutations tested so far that are amenable to rescue by SUs or CBZ are all in TMD0 (13, 15, 17, 19), a region known to mediate functional and physical interactions between SUR1 and Kir6.2 (20–22). Accordingly, we have found that the rescue effect of GBC and CBZ on TMD0 mutants is dependent on Kir6.2 and that both drugs promote the interaction between Kir6.2 and SUR1 (13, 23), suggesting that the pharmacological chaperones work in concert with Kir6.2 during translation and/or folding to overcome the structural defects imposed by the TMD0-SUR1 mutations. Importantly, mutants rescued to the cell surface by the reversible inhibitor CBZ or the reversible sulfonylurea tolbutamide often retain normal responses to ATP and MgADP upon drug washout (13, 15, 17), suggesting that these mutant channels may reestablish normal insulin secretion if surface expression is restored.
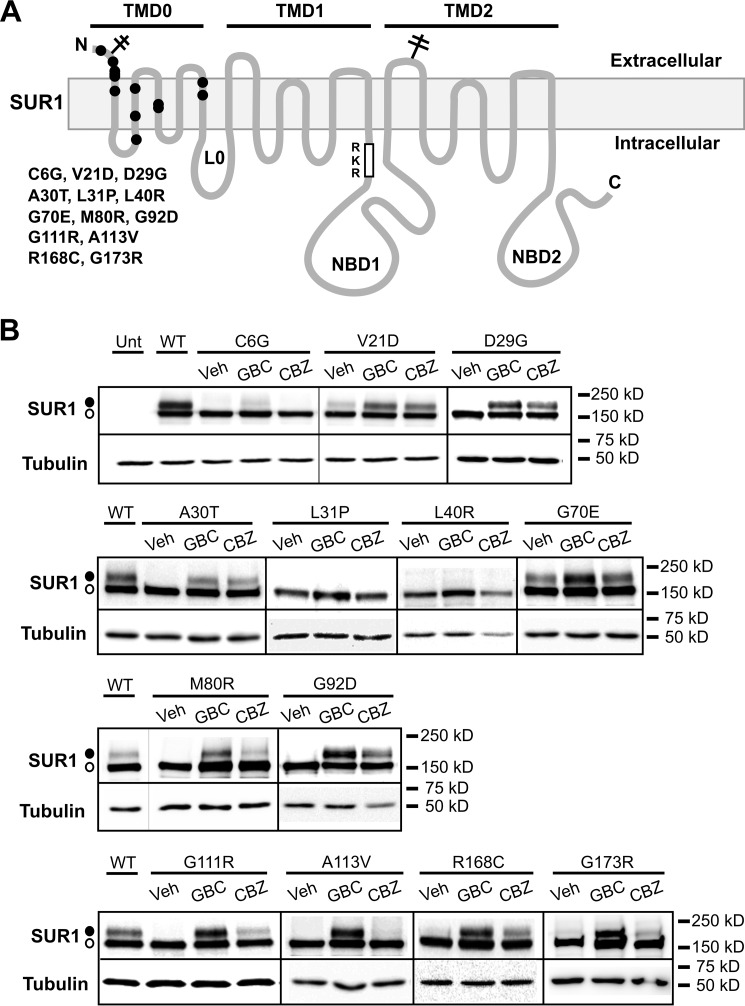
FIGURE 1.
Pharmacological correction of SUR1 processing defects caused by SUR1-TMD0 mutations identified in congenital hyperinsulinism. A, positions of SUR1 mutations included in this study are marked on a SUR1 topology model (29). B, Western blots of SUR1 from COSm6 cells co-transfected with human WT Kir6.2 and mutant SUR1 cDNA and treated with 0.1% DMSO (Veh), 5 μm GBC, or 10 μm CBZ for 16 h. Untransfected cells (Unt) and cells expressing WT channels were included for comparison. The thin lines separate different parts of the same blot, and thick vertical lines separate different blots. Empty circle, core-glycosylated immature SUR1; solid circle, complex-glycosylated mature SUR1. Molecular mass markers shown to the right of the blots are in kDa in this and all subsequent figures.
In the present study, we have identified and characterized 14 new, diazoxide-unresponsive HI mutations, each found within TMD0 of SUR1. We show that each of these mutations disrupts trafficking to the cell surface to varying degrees and that most are rescued by both GBC and CBZ. Within the group amenable to pharmacological chaperone rescue, most mutants exhibit normal responses to ATP and MgADP, and their functional recovery can be further facilitated by exposure to the KATP channel opener diazoxide. Interestingly, in contrast to KATP channel inhibitors, none of the several KATP channel openers that we tested showed chaperoning effects on the trafficking mutants. Further analysis using unnatural amino acid-mediated cross-linking showed that the opener diazoxide does not promote physical interactions between Kir6.2 and SUR1, in contrast to GBC or CBZ. The therapeutic and mechanistic implications of our findings broaden the applicability of pharmacological chaperones in treating HI.
Results
Identification and Pharmacological Rescue of Novel HI-causing KATP Trafficking Mutants
In a previous study (17), we identified a new KATP channel pharmacological chaperone CBZ which, like the sulfonylurea drugs glibenclamide and tolbutamide, can restore surface expression of several KATP trafficking mutants caused by mutations in the TMD0 region of SUR1. We sought to expand this finding by searching for additional HI-associated TMD0 mutations that disrupt channel trafficking and that are amenable to rescue by CBZ. Fourteen single nucleotide mutations in TMD0 identified by us or reported in the literature (11, 24) are included in this study, two of which resulted in the same amino acid change at position 111 from glycine to arginine, yielding a total of 13 missense mutations (Table 1; this number will be used hereinafter). The positions of these 13 mutations are shown in a topology map of SUR1 (Fig. 1A). Based on published data as well as our own genetic and clinical data, all 13 mutations are associated with diazoxide-unresponsive HI (Table 1). Of these mutations, only G70E and G111R have been subjected to biochemical and functional analysis (25), whereas channel defects caused by the other mutations are yet to be determined.
TABLE 1.
Genetic and clinical data of HI patients bearing the TMD0 mutations
| Exon | Nucleotide | Change | Codon | Amino acid change | Diazoxide-responsive | References |
|---|---|---|---|---|---|---|
| 1 | 16 | T → G | 6 | Cys → Gly | No | 42 |
| 1 | 62 | T → A | 21 | Val → Asp | No | 43 |
| 1 | 86 | A → G | 29 | Asp → Gly | No | 42 |
| 1 | 88 | G → A | 30 | Ala → Thr | No | 42 |
| 1 | 92 | T → C | 31 | Leu → Pro | No | 42 |
| 1 | 119 | T → G | 40 | Leu → Arg | No | 42 |
| 2 | 209 | G → A | 70 | Gly → Glu | No | 25 |
| 2 | 239 | T → G | 80 | Met → Arg | No | 44 |
| 2 | 275 | G → A | 92 | Gly → Asp | No | 45 |
| 3 | 331 | G → C | 111 | Gly → Arg | No | 46 |
| 3 | 331 | G → A | 111 | Gly → Arg | No | 25, 47, 48 |
| 3 | 338 | C → T | 113 | Ala → Val | No | 45 |
| 4 | 502 | C → T | 168 | Arg → Cys | No | 44 |
| 4 | 517 | G → A | 173 | Gly → Arg | No | 30 |
We first asked whether the mutations disrupt KATP channel biogenesis and whether CBZ can correct the adverse effects of the mutations, using a Western blot of SUR1 as a readout. SUR1 has two N-linked glycosylation sites that undergo core, high mannose glycosylation in the ER (12, 26). Upon assembly with Kir6.2 into octameric KATP channels and exit of the ER, SUR1 undergoes further glycosylation modifications in the Golgi before reaching the cell surface (22, 27). The core and complex glycosylated species can be separated by SDS-PAGE, producing a lower immature and a higher mature band, respectively. Because only fully assembled channels can pass ER quality control (22, 27), we can use the relative abundance of the upper and lower bands of SUR1 as an approximation for processing efficiency. COSm6 cells co-transfected with mutant SUR1 and WT Kir6.2 cDNAs (both human forms of the gene) were treated overnight (∼16 h) with vehicle control (0.1% DMSO), 5 μm GBC, or 10 μm CBZ followed by Western blotting of the whole-cell lysate. GBC was included as a comparison, because this sulfonylurea has been shown to be the most effective pharmacological chaperone for multiple TMD0 trafficking mutations (13, 15) and acts similarly to CBZ (23). WT SUR1/Kir6.2 was also included in each blot to serve as a positive control. Results from these experiments are shown in Fig. 1B. All 13 mutations reduced or diminished the upper SUR1 band. G70E and G111R have previously been reported to compromise channel biogenesis and trafficking (25), which are in agreement with our results. Cys-6 in SUR1 has been shown previously to form a disulfide bond with Cys-26, and mutation of Cys-6 to an alanine prevents maturation of the SUR1 band in cells co-expressing Kir6.2 (28). Our observation that C6G also disrupts SUR1 processing is consistent with the importance of this cysteine residue in channel biogenesis. Of the 13 trafficking mutations, L31P and L40R showed little or no response to either GBC or CBZ, and C6G responded only slightly to GBC and not at all to CBZ. The processing defects of the other mutations were rescued by both GBC and CBZ, although the response was variable, especially for CBZ rescue. Of note, we have previously shown that both GBC and CBZ also enhance the processing efficiency of WT channels, leading to a ∼5–10% increase in surface expression (13, 15, 17).
KATP Channel Openers Do Not Correct the Processing Defects of KATP Trafficking Mutants
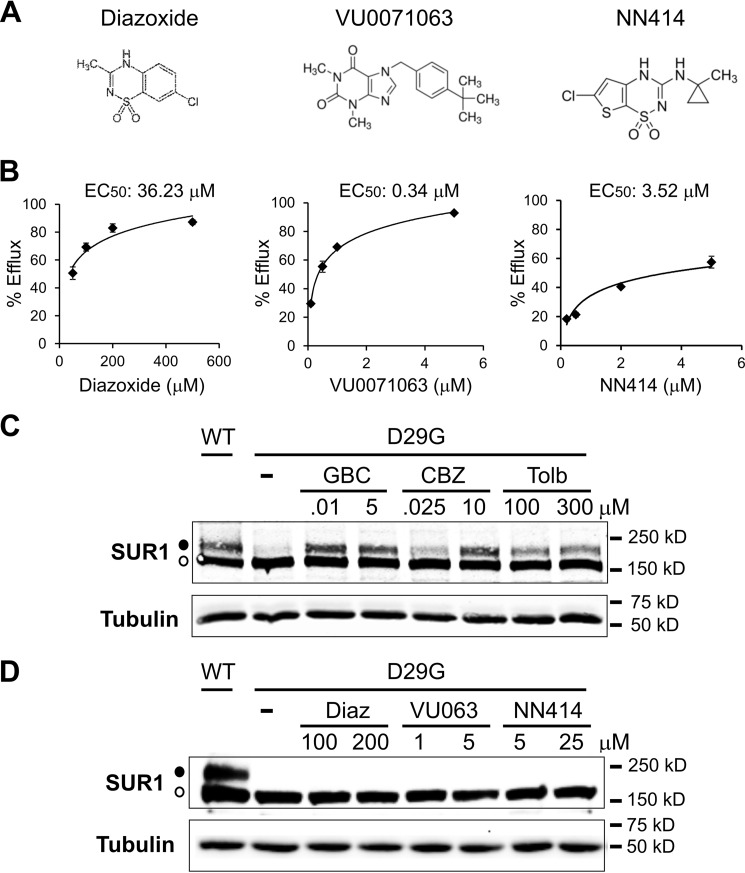
Our published studies have shown that whereas CBZ and sulfonylureas, such as GBC and tolbutamide, all inhibitors of the KATP channel, can act as pharmacological chaperones for a subset of KATP trafficking mutants, the KATP channel opener diazoxide (7-chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide) cannot (13, 15). We wondered whether the difference between channel inhibitors and openers with regard to rescuing trafficking-impaired KATP channels is generalizable. This is of interest because identification of a potassium channel opener that could act as a pharmacological chaperone for trafficking-impaired KATP channels would circumvent the problem of having to remove the inhibitory chaperones subsequently to recover channel function. To address this question, we tested several other compounds that have been reported to stimulate KATP channel activity, including VU0071063 (7-((4-(1,1-dimethylethyl)phenyl)methyl)-3,7-dihydro-1,3-dimethyl-1H-purine-2,6-dione) (31), Y26763 ((−)-(3S,4R)-4-(N-acetyl-N-hydroxyamino)-6-cyano-3,4-dihydro-2,2-dimethyl-2H-1-benzopyran-3-ol) (32), and NN414 (6-chloro-3-(1-methylcyclopropyl)amino-4H-thieno(3,2-e)-1,2,4-thiadiazine 1,1-dioxide) (33). Although EC50 values of these compounds for KATP channels have been reported or estimated previously (31–33), they were obtained using different experimental systems. We therefore wanted to determine each compound's EC50 under our experimental conditions. Channel activation was assessed by 86Rb+ efflux assays performed on cells transiently expressing the SUR1/Kir6.2 channels (see “Experimental Procedures”). In our system, we found that Y26763 is a poor activator of SUR1/Kir6.2 channels. At 25 μm (the EC50 provided by the vendor is 27 μm), Y26763 only gave a partial channel activation of ∼10% with an EC50 of ∼1.8 mm (data not shown). Therefore, we focused our efforts on VU0071063 and NN414. The chemical structures of VU0071063 and NN414 are shown in Fig. 2A, along with that of diazoxide. Based on dose-response curves of efflux activity normalized to that observed in cells treated with metabolic inhibitors, we calculated the EC50 of diazoxide, VU0071063, and NN414 to be 36.23, 0.34, and 3.52 μm, respectively (Fig. 2B). These results indicate that VU0071063 is the most potent KATP channel opener, followed by NN414 and then diazoxide. Interestingly, we also noted that the maximal efficacy of NN414 in this assay appeared lower than that of either diazoxide or VU0071063.
FIGURE 2.
KATP channel openers do not correct trafficking defects caused by SUR1 TMD0 mutations. A, chemical structures of diazoxide, VU0071063, and NN414 (from PubChem). B, dose-response curves of KATP channel stimulation by diazoxide, VU0071063, and NN414 in 86Rb+ efflux assays as described under “Experimental Procedures.” Percentage of efflux during a 40-min incubation period was derived by subtracting background efflux in untransfected cells and normalized to that observed in cells incubated with metabolic inhibitors. EC50 values were calculated by fitting the dose-response curve with an exponential equation using Excel. Each data point represents the mean ± S.E. (error bars) of three independent measurements. Note that the error bars for some data points are smaller than the size of the symbols and therefore not visible. C, Western blots of SUR1 from COSm6 cells co-transfected with WT Kir6.2 and WT or D29G SUR1 cDNA (both human clones) and treated with 0.1% DMSO (−), 0.01 or 5 μm GBC, 0.025 or 10 μm CBZ, or 100 or 300 μm tolbutamide (Tolb) for 16 h. In DMSO-treated sample, only the core-glycosylated lower immature SUR1 band was observed (empty circle). All three channel inhibitors corrected the processing defect of the D29G mutant at both concentrations, as evident by the appearance of the upper mature SUR1 band (solid circle). Cells expressing WT channels were included for comparison. D, same as C except cells were treated with two different concentrations of diazoxide (Diaz), VU0071063 (VU063), or NN414, as indicated. None of the KATP channel openers tested corrected the processing defect of the D29G mutant SUR1.
Having estimated the EC50 for the three KATP channel openers, we next tested each compound for its ability to rescue TMD0-SUR1 trafficking mutants, using the D29G as an example because it has the greatest response to GBC and CBZ. For each compound, we tested two different concentrations that were near (2–3-fold) or well above the EC50 but within solubility of the compound (5–12-fold). For comparison, cells transfected with the D29G mutant channel cDNAs were also treated overnight with GBC, CBZ, or tolbutamide at concentrations near or well above the reported IC50 values of each inhibitor (8, 23). In contrast to all three channel inhibitors (GBC, CBZ, and tolbutamide, which increased the upper band of the D29G SUR1 mutant at both concentrations (Fig. 2C)), none of the potassium channel openers showed any effects at either concentrations (Fig. 2D). The lack of a chaperoning effect of diazoxide has been reported for several other TMD0-SUR1 mutations (10, 13, 15), and our results here show that two other more potent channel openers also fail to correct trafficking defects of such mutants. These results indicate that in contrast to KATP channel inhibitors, KATP channel openers are unable to correct trafficking defects caused by SUR1-TMD0 mutations.
The KATP Channel Opener Diazoxide Does Not Enhance SUR1-Kir6.2 Subunit Interactions as Assessed by p-Azidophenylalanine-mediated Photocross-linking
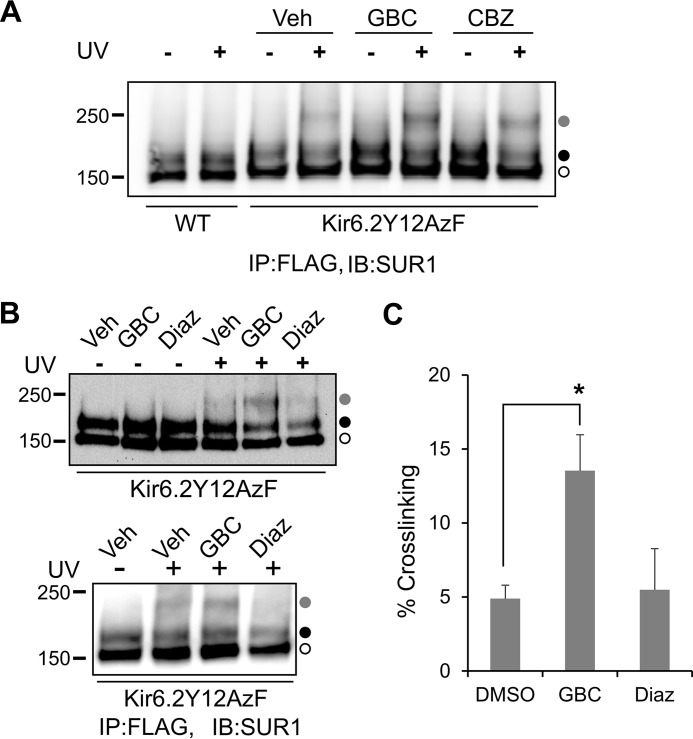
Using a genetically encoded photocross-linkable amino acid, p-azidophenylalanine, engineered into the distal N terminus of Kir6.2, we have recently shown that both GBC and CBZ promote cross-linking of the Kir6.2 N terminus to SUR1 (23). Based on this observation and findings that deleting the N terminus of Kir6.2 severely compromised the biogenesis efficiency of WT channels and prevented rescue of trafficking-impaired mutants by GBC or CBZ (23), we proposed that GBC and CBZ rescue TMD0-SUR1 trafficking mutants by promoting physical interactions between the N terminus of Kir6.2 and SUR1 to overcome channel protein folding and assembly defects caused by SUR1 TMD0 mutations. According to this model, KATP channel openers that do not correct channel trafficking defects should have no effect on the extent of photocross-linking between Kir6.2 N terminus and SUR1. To test this, we expressed FLAG-tagged SUR1 and a Kir6.2 variant carrying a TAG stop codon at amino acid position 12, as well as an orthogonal pair of tRNA and tRNA synthetase that incorporated p-azidophenylalanine (AzF) at the TAG stop codon position when cells were grown in culture medium containing the photocross-linkable unnatural amino acid, as described under “Experimental Procedures.” As shown in Fig. 3, A and B, whereas overnight treatment with GBC and CBZ increased the intensity of the cross-linked Kir6.2-SUR1 band compared with control cells treated overnight with 0.1% DMSO as reported previously (23), overnight treatment with 200 μm diazoxide did not. Quantification of the ratio of cross-linked band signal to total SUR1 signal from four independent experiments showed that GBC significantly increased the extent of cross-linking (13.54 ± 2.43% for GBC versus 4.88 ± 0.91% for DMSO control; p < 0.05), but diazoxide did not (5.49 ± 2.78% for diazoxide versus 4.88 ± 0.91% for DMSO control) (Fig. 3C). VU0071063 at 25 μm also failed to increase cross-linking between Kir6.2 and SUR1, although the results were less quantifiable because the drug also compromised the maturation of WT SUR1 (data not shown), possibly because it interferes with the association of Kir6.2 N terminus with SUR1 necessary for channel biogenesis and maturation, as we reported previously (23). Taken together, these results support the notion that compounds that do not rescue TMD0-SUR1 trafficking mutants also fail to promote the physical interaction between Kir6.2 N terminus and SUR1.
FIGURE 3.
Diazoxide does not promote intersubunit interactions between SUR1 and the N terminus of Kir6.2 as assessed by p-azidophenylalanine-mediated photocross-linking. A, COSm6 cells co-transfected with plasmids coding for azidophenylalanine tRNA, azidophenylalanine tRNA synthetase, WT hamster f-SUR1, and a rat Kir6.2 variant containing a stop codon at position 12 were grown in medium containing 1 mm azidophenylalanine and 0.1% DMSO (Veh), 5 μm GBC, or 10 μm CBZ for 36–48 h. Cells were harvested in cold PBS and subjected to photocross-linking as described under “Experimental Procedures.” Western blots of FLAG antibody affinity-purified SUR1 showed a cross-linked SUR1-Kir6.2 species (gray circle; confirmed by probing with anti-Kir6.2 antibody as we have reported previously (23), not shown) in addition to the lower (open circle) and upper (solid circle) SUR1 bands in cells expressing the Kir6.2Y12AzF variant and exposed to UV. The intensity of the cross-linked band was significantly higher in GBC- and CBZ-treated cells compared with DMSO-treated cells, as reported previously (23). Cells expressing WT Kir6.2 and WT f-SUR1 did not show the cross-linked species even with UV exposure, as expected. B, same as in A, except cells treated with DMSO, 5 μm GBC, or 200 μm diazoxide (Diaz) were compared. Two representative blots probed with anti-SUR1 (top) are shown. In both blots, GBC-treated cells showed an increased cross-linked band (gray circle) signal upon UV exposure compared with DMSO-treated controls. By contrast, diazoxide-treated cells did not show increased cross-linking compared with DMSO-treated controls. C, quantification of the intensity of the cross-linked band (as a percentage of total SUR1 signal) in the DMSO, GBC, and diazoxide groups. *, p < 0.05 by Student's t test, n = 4. Error bars, S.E.; IB, immunoblotting; IP, immunoprecipitation.
Trafficking-impaired KATP Channels Rescued by CBZ Reach the Cell Surface
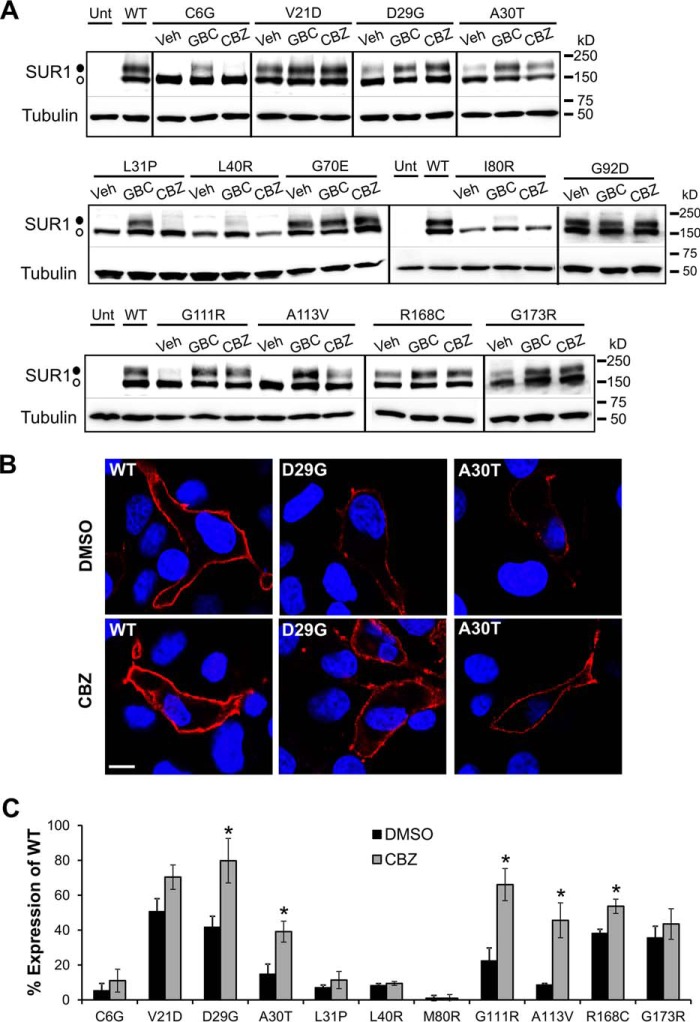
Having found that CBZ corrected the processing defects caused by the majority of the SUR1 TMD0 mutations, we directed our efforts toward further characterizing mutant channels rescued by the drug. As a first step, we performed immunostaining and quantitative immunochemiluminescence assays to confirm that the increased SUR1 upper band upon CBZ treatment corresponds to an increased expression of mutant channels at the cell surface. For these experiments, a higher signal/noise ratio is important for high quality results; we therefore used hamster SUR1/rat Kir6.2 recombinant channels because these channels express at a higher level compared with human channels (15, 17, 34). Western blotting experiments like those done for human mutant channels were repeated in cells co-expressing hamster SUR1 with corresponding mutations and WT rat Kir6.2. By and large, the hamster SUR1/rat Kir6.2 mutant channels responded to GBC and CBZ similarly to their human counterparts (Fig. 4A). However, some differences are noted. For example, most mutants showed better processing efficiency even without any drug treatment; this is particularly true for G92D. Also, C6G, L31P, and L40R had better response to GBC compared with their human counterparts.
FIGURE 4.
Carbamazepine restores surface expression of trafficking-impaired SUR1 mutants. A, Western blots of f-SUR1 from COSm6 cells co-transfected with rat WT Kir6.2 and mutant hamster f-SUR1 cDNA and treated with 0.1% DMSO (Veh), 5 μm GBC, or 10 μm CBZ for 16 h. Note that in hamster SUR1, amino acid position 80 is an isoleucine rather than a methionine found in human SUR1. Untransfected cells (Unt) and cells expressing WT channels were included for comparison. The thin lines separate different parts of the same blot, and thick vertical lines separate different blots. The empty circle points to the core-glycosylated immature SUR1, and the solid circle points to the complex-glycosylated mature SUR1. B, surface expression of the D29G or A30T SUR1 mutants in cells transfected with WT rat Kir6.2 and hamster D29G or A30T f-SUR1 was monitored by immunostaining of the extracellular FLAG epitope tag of f-SUR1 (red) in non-permeabilized cells. The nuclei were stained with DAPI (blue). Scale bar, 5 μm. WT channels were included for comparison. The staining was repeated twice with qualitatively similar results. C, quantification of surface expression rescue of trafficking-impaired KATP channels by chemiluminescence assays. Each bar represents the mean ± S.E. (error bars) of 3–4 experiments. *, p < 0.05 comparing DMSO (0.1%)-treated versus CBZ-treated (10 μm) cells by Student's t test. Note that although the average surface expression level for all mutants was higher in CBZ-treated cells compared with DMSO-treated cells, the increase did not reach statistical significance for some of the mutants.
To track KATP channel surface expression, we used a SUR1 construct containing an N-terminal FLAG tag located at the extracellular N terminus (FLAG-SUR1). When staining cells that have not been fixed and permeabilized, only surface FLAG-SUR1 is accessible to labeling by anti-FLAG antibody present in the medium. Fig. 4B shows representative examples (D29G and A30T). Comparing surface staining of WT and D29G- or A30T-SUR1 with or without overnight CBZ treatment, CBZ had a clear effect on the mutant channels, with generally more cells showing more intense surface staining following treatment. Quantitative surface chemiluminescence experiments (see “Experimental Procedures”) were further performed on the mutants (excluding hamster G70E and G92D, which showed WT-like processing efficiency even without GBC or CBZ treatment; see Fig. 4A). Results from these experiments (Fig. 4C) correlate well with immunoblotting and staining results and further confirm the efficacy of CBZ in rescuing this class of trafficking mutations to the cell surface.
Gating Properties of Mutant Channels Rescued to the Cell Surface
KATP channels are gated by intracellular adenine nucleotides; ATP binds to Kir6.2 in a Mg2+-independent manner to block channel activity, whereas MgATP binds to SUR1 to stimulate channel activity (9, 35). At low glucose concentrations, MgADP stimulation of KATP channels dominates, producing K+ efflux that keeps the β-cell hyperpolarized and prevents insulin secretion. When blood glucose concentrations rise, the β-cell intracellular ATP concentration also rises, leading to KATP channel inhibition, membrane depolarization, and insulin secretion. The ability of the channel to respond to ATP inhibition and MgADP stimulation is critical for its physiological function to ensure correct insulin secretion response (7, 36, 37). In addition, diazoxide is a potassium channel opener that can be used to enhance KATP channel activity to suppress insulin secretion and is clinically effective in treating HI patients with residual channel function (5). Because some trafficking mutations have been shown to cause both trafficking and gating defects (16, 38, 39), it is important to ascertain that trafficking-impaired channels rescued to the cell surface are gated properly by ATP, MgADP, and diazoxide. To this end, we performed inside-out patch clamp recording using COSm6 cells transfected with mutant channels and treated overnight with CBZ. We have previously shown that CBZ inhibits channel activity (17), specifically abolishing channel response to MgADP (18), and this effect is reversible (17, 18). Therefore, recordings were made after CBZ was thoroughly washed out, by incubating cells in CBZ-free medium for 2 h, to unmask the gating properties of rescued channels. Also, because C6G, L31P, and L40R showed little rescue by CBZ, these mutations were excluded from our analysis.
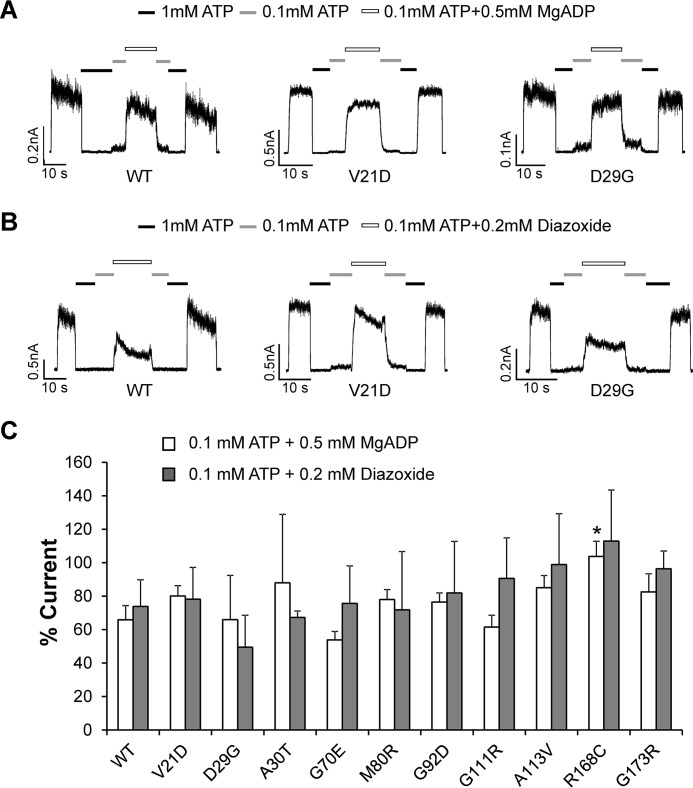
All mutants tested had detectable currents after CBZ rescue and washout. Current traces from two mutants, V21D and D29G, in response to ATP and MgADP or ATP and diazoxide are shown in Fig. 5, A and B, as examples. Quantification of MgADP and diazoxide sensitivity for all mutants, expressed as the percentage of current relative to the nucleotide-free bath solution, is shown in Fig. 5C. The results show that sensitivities of these mutants to MgADP or diazoxide stimulation were not significantly different from those of WT channels, with the exception of R168C, which showed a small increase in sensitivity to MgADP (p = 0.03). The results suggest that upon rescue to the cell surface, these mutant channels will open in response to metabolic inhibition or diazoxide stimulation to suppress insulin secretion.
FIGURE 5.
Gating properties of mutant channels rescued to the cell surface assessed by inside-out patch-clamp recording. COSm6 cells expressing the various trafficking mutants (human SUR1 + human Kir6.2) were treated overnight with 10 μm CBZ to rescue channels to the cell surface. CBZ was removed from the culture medium for at least 2 h before subjecting cells to inside-out patch clamp recording, as described under “Experimental Procedures.” A, representative current traces from WT, V21D, and D29G channels exposed to K-INT solution with or without ATP and MgADP, as indicated. B, current traces from WT, V21D, and D29G exposed to K-INT solution with or without ATP and diazoxide, as indicated. For both A and B, currents were recorded at −50 mV, and inward currents are shown as upward deflections. C, quantification of channel response to 0.1 mm ATP + 0.5 mm MgADP or 0.1 mm ATP + 0.2 mm diazoxide. Currents were normalized to those observed in K-INT only. Each bar represents mean ± S.E. (error bars) (n = 17–18 for WT MgADP, n = 6 for WT diazoxide, n = 3–8 for mutants). *, p < 0.05 (R168C versus WT MgADP response by Student's t test).
Assessing Functional Recovery of Mutant Channels Rescued to the Cell Surface in Intact Cells
To directly test whether CBZ-rescued channels can respond to metabolic signals, we performed 86Rb+ efflux assays, which assess channel activity in intact cells. COSm6 cells were transiently transfected with each of the 10 different trafficking mutants that showed response to CBZ rescue in Fig. 1B. Cells were then treated overnight with 10 μm CBZ and subjected to 86Rb+ efflux assays, as described under “Experimental Procedures.” To mimic hypoglycemic conditions that activate the channels, cells were preincubated with metabolic inhibitors in Ringer's solution for 30 min, and efflux was monitored during a 40-min period in Ringer's solution with metabolic inhibitors. CBZ was not present in the metabolic inhibitor preincubation solution or the efflux solution to allow CBZ to unbind, thus removing channel inhibition. In parallel, we also included experimental groups in which the KATP channel opener diazoxide or VU0071063 was included (see the schematic in Fig. 6A). We chose diazoxide and VU0071063 because the former has previously been shown to facilitate functional recovery of CBZ-rescued channels in another TMD0-SUR1 trafficking mutation, F27S (17), and VU0071063 is the most potent and effective WT KATP channel opener among the channel openers that we tested in this study. These experiments revealed differential functional recovery for the various mutants and differential effects of the two KATP channel openers on functional recovery of CBZ-rescued mutant channels (Fig. 6B).
FIGURE 6.
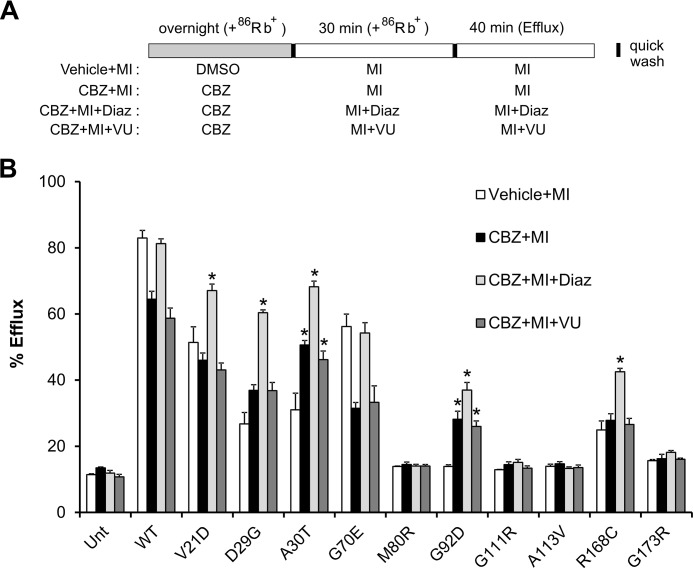
Assessing metabolic response of mutant KATP channels rescued by CBZ using 86Rb+ efflux assays. A, schematic of experimental design. COSm6 cells transfected with WT or various mutant channels (human clones) were treated with 0.1% DMSO (vehicle control) or 10 μm CBZ overnight in the presence of 86Rb+. Before efflux measurements, cells were incubated with metabolic inhibitors (1 mm deoxyglucose and 2.5 μg/ml oligomycin) to activate channels at the cell surface; 86Rb+ was included during this time to maintain 86Rb+ loading. During the 30-min metabolic inhibition (MI) period, CBZ was not included to wash out CBZ bound to rescued channels, whereas 200 μm diazoxide or 25 μm VU063 was included to test their effects on facilitating CBZ removal. Efflux was then measured for 40 min in the same solutions as during metabolic inhibition but in the absence of 86Rb+. B, efflux over a 40-min period is expressed as a percentage of total 86Rb+ counts. Untreated untransfected cells and cells transfected with WT channels were included as controls. Each bar represents mean ± S.E. (error bars) of three independent experiments. *, significant increase comparing CBZ + MI, CBZ + MI + Diaz, or CBZ + MI + VU with Vehicle + MI (p < 0.05 by one-way ANOVA and Dunnett's post hoc test).
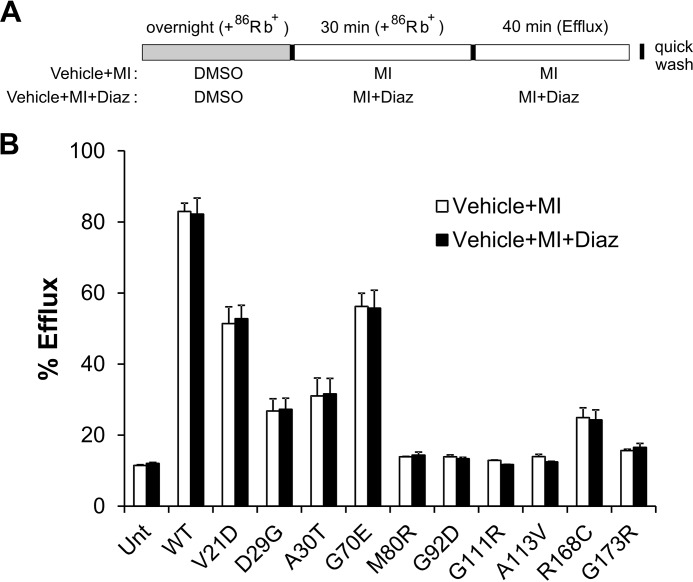
Upon CBZ overnight treatment followed by CBZ washout (total washout of 70 min, including the 30-min metabolic inhibition and the 40-min efflux period), several mutations showed significantly more efflux activity in response to metabolic inhibition compared with cells treated overnight with the vehicle (DMSO) control; these include D29G, A30T, and G92D (Fig. 6B). Two mutations, V21D and G70E, actually showed decreased efflux activity compared with vehicle-treated controls, which was also observed in WT channels, indicating that CBZ had not been completely washed out. It is interesting to point out that both V21D and G70E only had mild trafficking defects, such that CBZ overnight treatment probably only had a small effect on channel numbers at the cell surface, and this effect was masked by the inhibitory effect of CBZ on channel gating. Other mutations, including M80R, G111R, A113V, R168C, and G173R, showed only a very small (not statistically significant) increase in efflux activity. When 200 μm diazoxide was included in the metabolic inhibitor preincubation and efflux solutions, an additional amount of significant functional recovery was observed in D29G, A30T, G92D, and R168C mutants (Fig. 6B). Note that this increase in efflux activity was not due to additional stimulation of channel activity by diazoxide beyond that stimulated by metabolic inhibition but actually reflects recovery of function of CBZ-rescued mutant channels. This conclusion is based on experiments comparing the efflux activity of cells expressing trafficking mutants, treated with the vehicle DMSO (vehicle), and then subjected to metabolic inhibition in the absence or presence of diazoxide, which showed no difference whether diazoxide was present or not (Fig. 7). In V21D, the addition of diazoxide enhanced efflux activity, whereas in G70E, diazoxide recovered efflux that was inhibited by residually bound CBZ. For M80R, G111R, A113V, and G173R, diazoxide did not improve efflux activity significantly. To our surprise, inclusion of 25 μm VU0071063, a concentration that maximally stimulated WT channels similar to metabolic inhibition, did not facilitate functional recovery of CBZ-rescued mutant channels (Fig. 6B), in contrast to what was observed for diazoxide.
FIGURE 7.
Diazoxide does not further increase 86Rb+ efflux by increasing activity of channels already activated by metabolic inhibition. A, schematic of the rubidium efflux experiment. COSm6 cells expressing WT or various mutant channels were loaded with 86Rb+ overnight without CBZ rescue (only 0.1% DMSO vehicle was added to the medium). Metabolic inhibition and efflux were carried out in the presence of 200 μm diazoxide. B, efflux over a 40-min period is expressed as percentage of total 86Rb+ counts. Note that diazoxide did not significantly enhance efflux activity of WT or mutant channels present in the membrane, suggesting that existing channels were already maximally activated by metabolic inhibition. Each bar represents mean ± S.E. (error bars) of three independent experiments. No statistically significant difference was found between the two treatment groups in all mutants by Student's t test.
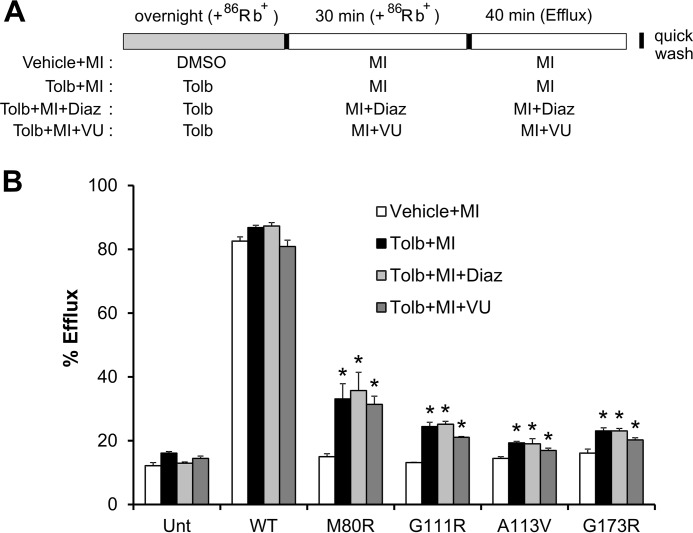
Results shown in Fig. 6 suggest that CBZ was not completely removed during the efflux experiment. Therefore, we repeated the efflux experiments for mutants that showed little functional rescue effect, specifically M80R, G111R, A113V, and G173R, using a different pharmacological chaperone, tolbutamide (Fig. 8A). Tolbutamide is a low affinity sulfonylurea drug that has been shown in our previous studies to rescue TMD0 trafficking mutations (13, 15). Although it is less effective than the irreversible sulfonylurea GBC in rescuing trafficking defects caused by TMD0 mutations, it is easily washed out to recover the function of rescued channels. Indeed, as shown in Fig. 8B, the four mutants did show small but significant increases in efflux activity upon tolbutamide washout, demonstrating the surface expression rescue effect of tolbutamide and removal of tolbutamide during washout. The small improvement in functional recovery observed in these mutants is consistent with the extent of CBZ rescue observed in Western blotting experiments (Fig. 1B). Of note, diazoxide had very small or negligible effects in further increasing the efflux activity. The results suggest that tolbutamide was washed out efficiently such that diazoxide did little to facilitate unbinding of the channel inhibitor. In conclusion, using tolbutamide or CBZ in combination with diazoxide, most trafficking mutants showed functional recovery in response to metabolic signals.
FIGURE 8.
Metabolic response of mutant KATP channels rescued by tolbutamide assessed by 86Rb+ efflux assays. A, schematic of experimental design as in Fig. 6A except that a 300 μm tolbutamide overnight treatment was used to rescue mutant channels. B, efflux of tested mutants over a 40-min period is expressed as percentage of total 86Rb+ counts. Each bar represents mean ± S.E. (error bars) of three independent experiments. *, significant increase comparing Tolb + MI, Tolb + MI + Diaz, or Tolb + MI + VU with Vehicle + MI (p < 0.05 by one-way ANOVA and Dunnett's post hoc test).
Discussion
HI is a rare disease, with loss of function mutations in the KATP channel genes, ABCC8 and KCNJ11, accounting for more than ∼50% of the cases (5). Patients with mutations that impair trafficking of KATP channels to the cell surface often present with severe disease phenotypes that are not responsive to diazoxide treatment, requiring pancreatectomy to prevent life-threatening hypoglycemia (5, 11). Pharmacological chaperone therapy represents a promising noninvasive treatment approach to this group of patients. However, unlike cystic fibrosis, in which ∼90% patients carry one or two copies of a highly prevalent misfolding mutation, ΔF508, in the cystic fibrosis transmembrane conductance regulator protein (40), there is no single highly prevalent mutation in the KATP channel genes that underlies channel trafficking defects. Instead, HI-associated channel trafficking mutations are rare mutations scattered throughout the two channel proteins (10, 11), making it challenging to identify small molecules that can be applied to most patients.
Our previous studies have shown that several compounds that bind to the channel and inhibit channel activity are able to correct trafficking defects of KATP channels (13–15, 17, 19). These include two classes of oral hypoglycemic agents: sulfonylureas and glinides, with tolbutamide and glibenclamide in the former class (13, 15) and rapaglinide the latter (14), as well as the anticonvulsant carbamazepine that we reported recently (17, 19). Interestingly, these earlier studies found that of >20 trafficking mutations scattered throughout the SUR1 protein tested, only those located in the TMD0 domain were rescued. The emerging pattern led us to focus in the current study on searching for more TMD0 trafficking mutations that are amenable to pharmacological rescue by KATP channel inhibitors. Such a targeted search led to the identification of 10 SUR1-TMD0 missense trafficking mutations that responded to CBZ rescue as determined by Western blotting. All of these mutations had detectable currents and exhibited WT-like gating properties upon washout of CBZ in patch-clamp recordings. These results expand the list of mutant candidates amenable to pharmacological chaperone therapy from the previously identified 7 mutations to now 17 mutations. Expansion of this list is highly significant for a rare disease like HI because it provides the impetus for future efforts toward developing pharmacological chaperones for clinical application.
Among the 13 SUR1 mutations tested, L31P and L40R showed no trafficking rescue by either GBC or CBZ in any assay tested, whereas C6G showed only a barely detectable increase in the upper band in response to GBC but not with CBZ in Western blots. The other mutations that responded to both GBC and CBZ also exhibited variable rescue. The lack of or weak response of some mutants could be due to an inability or reduced affinity of the mutant proteins to bind GBC or CBZ because of mutation-induced misfolding. Alternatively, the drug binding pockets might remain unaffected in the mutant proteins but drug binding fails to overcome channel biogenesis/trafficking defects induced by these mutations due to mutation-dependent structural constraints. Detailed analysis of binding affinities to GBC and CBZ of the various mutants will be needed in the future to answer these question.
One of the major barriers to using KATP channel inhibitors for pharmacological chaperone therapy is the need to remove the inhibitors once mutant channels are rescued to the cell surface to recover the function of rescued channels (13, 15, 17). For this reason, we tested several KATP channel openers reported in the literature in hopes of identifying ligands that will both correct channel trafficking defects and boost channel function despite our previous studies showing that the most widely used KATP channel opener diazoxide failed to rescue TMD0 trafficking mutations examined (13, 15, 17). Our experimental results showed that none of the four other KATP channel openers tested corrected the processing defects of TMD0 mutants, over a wide range of concentrations, regardless of the potency or efficacy of the opener estimated from 86Rb+ efflux assays. Thus, the contrast between channel inhibitors and openers with regard to their ability to correct biogenesis/trafficking defects of TMD0-SUR1 mutations appears generalizable. Interestingly, using an unnatural amino acid-mediated photocross-linking experimental paradigm that we developed previously to probe GBC- or CBZ-induced conformational change in KATP channels (23), we found that, in contrast to GBC and CBZ, both of which significantly increased cross-linking between an AzF placed at the 12th amino acid position in the distal N-terminal region of Kir6.2 and SUR1 (Fig. 3A) (23), diazoxide did not. These findings support a mechanistic model in which channel inhibitors correct TMD0 mutation-induced channel biogenesis/trafficking defects by promoting interactions between the N terminus of Kir6.2 and SUR1, whereas openers do not correct defects in these mutations because they do not enhance the physical interactions/assembly between the Kir6.2 N terminus and SUR1. This model is consistent with a channel gating model proposed previously (20), in which the N terminus of Kir6.2 interacts with the cytoplasmic loop following TMD0 of SUR1 known as L0 (see Fig. 1A) to modulate channel open probability. A possible scenario is that channel inhibitors, such as GBC and CBZ, reduce channel open probability, at least in part, by increasing the probability of interaction between the Kir6.2 N terminus and SUR1-L0 to decrease channel open probability, whereas channel agonists, such as diazoxide, stabilize the channel in a conformation that prevents the N terminus of Kir6.2 from interacting with L0 of SUR1 to increase channel open probability. Identification of residues in SUR1 to which the AzF in Kir6.2 is cross-linked will provide a rigorous test of this hypothesis.
Of the KATP inhibitors that exhibit chaperoning effects on TMD0 trafficking mutants, GBC is the most potent and effective; however, its high binding affinity for the channel makes it nearly impossible to wash out (13). The glinide drug rapaglinide also resulted in nearly irreversible inhibition even after removal of the drug from the culture medium (14). By contrast, CBZ and tolbutamide could be washed out to allow channel function recovery. Our previous and present studies indicate, however, that complete removal of CBZ inhibition requires extensive washout longer than the efflux experimental duration (Fig. 6A). This is based on the lower efflux activity observed in the CBZ washout group compared with the vehicle-treated control group for WT channels and several mutant channels that exhibit higher levels of basal surface expression, such as V21D and G70E (Fig. 6B). Removal of CBZ inhibition was facilitated by the addition of diazoxide, which was especially evident in trafficking mutants that showed a significant increase in surface expression upon CBZ treatment, such as D29G, A30T, and G92D. To our surprise, VU0071063, which is a more potent KATP channel opener than diazoxide (Fig. 2B) (31), did not facilitate functional recovery of CBZ-rescued channels. These findings suggest that diazoxide and VU0071063 may interact with the KATP channel via different sites and/or with different kinetics, such that only diazoxide can efficiently remove residually bound CBZ. Finally, for several mutations that only showed a weak response to CBZ rescue in Western blots and little improvement in efflux activity, we were able to observe greater functional rescue when tolbutamide was used as the pharmacological chaperone; in this case, diazoxide did not further improve efflux activity in these mutants. These results can be explained by the much lower binding affinity of tolbutamide and hence the higher efficiency of drug washout.
In summary, our present study further strengthens the rationale of repurposing CBZ and tolbutamide to treat HI caused by TMD0 trafficking mutations. The choice of which reversible channel inhibitor to use and whether diazoxide can further improve functional recovery may depend on the mutation. Biochemical and functional characterizations of mutation response like those presented here will help with this decision. It is important to point out that although functional recovery in some mutants is small, such small improvement may be sufficient to avoid severe HI (32). In addition, our study provides further insight into a structural mechanism by which KATP channel inhibitors do, whereas openers do not, rescue SUR1-TMD0 trafficking mutants. The improved mechanistic understanding of pharmacological chaperoning will be helpful in designing more efficient ligands to manipulate KATP surface expression and function for disease treatment.
Experimental Procedures
Genetic and Clinical Studies
The subjects included in this study were patients referred to the Children's Hospital of Philadelphia Congenital Hyperinsulinism Center or reported by others in the literature (see Table 1). Patients were defined as being unresponsive to diazoxide if hypoglycemia could not be controlled by treatment with 15 mg/kg/day diazoxide for a minimum of 5 days (i.e. able to keep blood glucose >70 mg/dl for >8–10 h of fasting). Most of these diazoxide-unresponsive patients required surgical pancreatectomy. Clinical information was abstracted from the medical records. Written informed consent was obtained from parents of the probands included in this study. The study was reviewed and approved by the Institutional Review Board of the Children's Hospital of Philadelphia.
For genetic analysis, peripheral blood was obtained from patients for isolation of genomic DNA (5 PRIME, Gaithersburg, MD). Coding sequences and intron/exon splice junctions were amplified and directly sequenced on an ABI 3730 capillary DNA analyzer (Applied Biosystems, Carlsbad, CA). The nucleotides of ABCC8 and corresponding SUR1 amino acids were numbered according to the sequence reported by Nestorowicz et al. (41), which includes the alternatively spliced exon 17 sequence (NCBI accession number L78224).
Molecular Biology
For the majority of the experiments, human SUR1 cDNA in pCMV6b (kindly provided by Dr. Joseph Bryan) and human Kir6.2 in pcDNA3.1 were used. Point mutations were introduced using the QuikChange site-directed mutagenesis kit (Stratagene). For immunostaining and chemiluminescence experiments, rat Kir6.2 in pcDNA1 and hamster FLAG epitope-tagged SUR1 (referred to as f-SUR1) in pECE were used. The FLAG epitope (DYKDDDDK) was inserted at the N terminus of the hamster SUR1 cDNA, as described previously (38). We have demonstrated in previous studies (13, 15, 38) that the FLAG epitope placed at the extracellular N terminus of SUR1 does not affect channel assembly or function. All mutations were confirmed by DNA sequencing, and mutant clones from two independent PCRs were analyzed in all experiments to avoid false results caused by undesired mutations introduced by PCR.
Immunoblotting
COSm6 cells were transfected with SUR1 and Kir6.2 using FuGENE®6 and lysed in 20 mm HEPES, pH 7.0, 5 mm EDTA, 150 mm NaCl, 1% Nonidet P-40 with CompleteTR protease inhibitors (Roche Applied Science) 48–72 h post-transfection. Proteins in cell lysates were separated by SDS-PAGE (8%), transferred to nitrocellulose membrane, analyzed by M2 anti-FLAG antibody followed by HRP-conjugated anti-mouse secondary antibodies (Amersham Biosciences) and visualized by chemiluminescence (Super Signal West Femto, Pierce) with FluorChem E (ProteinSimple).
Immunofluorescence Staining
COSm6 cells were grown on coverslips and transfected with hamster f-SUR1 and rat Kir6.2. Cells were treated with 0.1% DMSO or 10 μm carbamazepine 32–40 h post-transfection overnight (∼16 h) and then processed for immunofluorescence staining. To stain for surface KATP channels, cells were incubated with anti-FLAG M2 mouse monoclonal antibody (Sigma; diluted to 10 μg/ml in Opti-MEM containing 0.1% BSA) for 1 h at 4 °C to detect f-SUR1, washed with ice-cold PBS, and then fixed with −20 °C methanol for 10 min. Fixed cells were incubated with Cy-3 conjugated donkey anti-mouse secondary antibodies (Jackson) for 30 min at room temperature, followed by three 5-min washes in PBS. Coverslips were mounted on glass slides using Vectashield mounting medium for fluorescence with DAPI to counterstain the nuclei. Cells were viewed using an Olympus FV1000 laser-scanning confocal microscope.
Chemiluminescence Assays
COSm6 cells were plated on 35-mm dishes and transfected with cDNAs for the KATP channel subunits using FuGENE®6. Drug treatment was carried out 32–40 h post-transfection and lasted for ∼16 h. Cells were then processed for a chemiluminescence assay, as described previously (15). In brief, cells were fixed with 2% paraformaldehyde for 30 min at 4 °C, preblocked in PBS + 0.1% BSA for 30 min, incubated in M2 anti-FLAG antibody (10 μg/ml) for 1 h, washed for 30 min in PBS + 0.1% BSA four times, incubated in HRP-conjugated anti-mouse antibody (Jackson; 1:1000 dilution) for 20 min, and washed for 30 min in PBS + 0.1% BSA four times. Peak chemiluminescence signal of each dish was quantified in a TD-20/20 luminometer (Turner Designs) following a 5-s incubation in Power Signal Elisa Femto luminol solution (Pierce). All steps after fixation were carried out at room temperature. For data quantification, chemiluminescence signal in untransfected cells was subtracted as background, which was typically <10% of total signal observed in cells transfected with WT channels. The signal from each mutant with or without CBZ treatment was then normalized to that of WT channels.
86Rb+ Efflux Assays
Transfected COSm6 cells in 12-well plates were incubated overnight in medium containing 86RbCl (0.1 μCi/ml) with or without 10 μm carbamazepine or 300 μm tolbutamide, as specified. Cells were washed in Krebs-Ringer solution twice and incubated with metabolic inhibitors (2.5 μg/ml oligomycin and 1 mm 2-deoxy-d-glucose) in Krebs-Ringer solution for 30 min in the presence of 86Rb+ without carbamazepine but with or without 200 μm diazoxide or 10 μm VU0071063. Following two quick washes in efflux solution (as specified in Fig. 6), 0.5 ml of efflux solution was added to each well and incubated for 40 min. At the end of the 40-min incubation, solution was collected, and cells were lysed in Krebs-Ringer containing 1% SDS. 86Rb+ in the solution and the cell lysate was counted. The percentage efflux was calculated as the radioactivity in the efflux solution divided by the total activity from the solution and cell lysate, as described previously (15, 17).
Patch Clamp Recordings
COSm6 cells were transfected using FuGENE®6 and plated onto coverslips. The cDNA for the GFP was co-transfected with SUR1 and Kir6.2 to facilitate identification of transfected cells. Patch clamp recordings were made 36–72 h post-transfection. All experiments were performed at room temperature, as described previously (15). Micropipettes were pulled from non-heparinized Kimble glass (Fisher) on a horizontal puller (Sutter Instrument Co., Novato, CA). Electrode resistance was typically 1–2 megaohms when filled with K-INT solution (see below). Inside-out patches were voltage-clamped with an Axopatch 1D amplifier (Axon Inc., Foster City, CA). The standard bath (intracellular) and pipette (extracellular) solution (K-INT) had the following composition: 140 mm KCl, 10 mm K-HEPES, 1 mm K-EGTA, pH 7.3. ATP was added as the potassium salt. All currents were measured at a membrane potential of −50 mV (pipette voltage = +50 mV). Data were analyzed using pCLAMP10 software (Axon Instruments). Off-line analysis was performed using Microsoft Excel. Data are presented as means ± S.E.
Construction, Expression, and UV-induced Photocross-linking of Kir6.2 with Genetically Encoded p-Azido-l-phenylalanine
COSm6 cells were co-transfected with plasmids coding for azidophenylalanine tRNA, azidophenylalanine tRNA synthetase, WT SUR1, and Kir6.2 containing stop codons at position 12 using FuGENE®6 as described previously (23). Growth medium was supplemented with 0.5–1 mm azidophenylalanine 12 h after transfection, and cells were grown for 36–48 h with or without KATP channel inhibitors or openers, as specified. Cells were harvested in cold PBS, incubated with drugs for 10 min at 37 °C, and exposed to UV for 15 min. Cells were pelleted and lysed in lysis buffer (20 mm HEPES, pH 7.2, 125 mm NaCl, 4 mm EDTA, 1 mm EGTA, 1% Triton X-100) with complete protease inhibitors for 30 min and centrifuged for 15 min in a table top centrifuge; supernatant was collected and incubated with anti-FLAG antibody-conjugated agarose beads overnight and washed three times with 1 ml of a buffer containing 20 mm HEPES, pH 7.2, 150 mm NaCl, 4 mm EDTA, 1 mm EGTA, 1% Nonidet P-40, 0.1% SDS, and 0.04% deoxycholic acid; and finally, bound proteins were eluted with 1% SDS, run on 3–8% SDS Tris acetate gels, and subjected to Western blotting analysis.
Statistics
Data are presented as mean ± S.E. Differences were tested using analysis of variance (ANOVA) when comparing three or more groups, and Dunnett's post hoc test was used to compare the treated versus control group. When only two groups were compared, unpaired Student's t tests were used. Differences were considered significant if p was ≤0.05.
Author Contributions
G. M. M., E. A. R., P. D., and S.-L. S. designed and conducted experiments and wrote the manuscript. J. S. D. provided expert help on using the potassium channel potentiators. K. E. B., C. A. S., and D. D. D. collected genetic and clinical data from patients.
Acknowledgments
We thank Erik Olson for technical help, Dr. Yelena Kryukova for sharing data, and Dr. Pei-Chun Chen for helpful discussion. We also thank Dr. Dale Fortin, Dr. Yi Wu, and Veronica Cochrane for critical reading of the manuscript.
This work was supported by National Institutes of Health Grants R01DK066485 (to S.-L. S.), F31DK105800 (to G. M. M.), R01DK098517 (to D. D. D. L.), and R37DK056268 (to C. A. S.) and a grant from the Goldsmith Foundation (to D. D. D. L. and C. A. S.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- ER
- endoplasmic reticulum
- HI
- hyperinsulinism
- ATP
- sensitive potassium
- ABC
- ATP-binding cassette
- SU
- sulfonylurea
- GBC
- glibenclamide
- CBZ
- carbamazepine
- AzF
- p-azidophenylalanine
- ANOVA
- analysis of variance
- MI
- metabolic inhibition
- KATP
- ATP-sensitive potassium.
References
- 1. Welch W. J. (2004) Role of quality control pathways in human diseases involving protein misfolding. Semin. Cell Dev. Biol. 15, 31–38 [DOI] [PubMed] [Google Scholar]
- 2. Convertino M., Das J., and Dokholyan N. V. (2016) Pharmacological chaperones: design and development of new therapeutic strategies for the treatment of conformational diseases. ACS Chem. Biol. 11, 1471–1489 [DOI] [PubMed] [Google Scholar]
- 3. Leidenheimer N. J., and Ryder K. G. (2014) Pharmacological chaperoning: a primer on mechanism and pharmacology. Pharmacol. Res. 83, 10–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powers E. T., Morimoto R. I., Dillin A., Kelly J. W., and Balch W. E. (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959–991 [DOI] [PubMed] [Google Scholar]
- 5. Stanley C. A. (2016) Perspective on the genetics and diagnosis of congenital hyperinsulinism disorders. J. Clin. Endocrinol. Metab. 101, 815–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aguilar-Bryan L., Bryan J., and Nakazaki M. (2001) Of mice and men: K(ATP) channels and insulin secretion. Recent Prog. Horm. Res. 56, 47–68 [DOI] [PubMed] [Google Scholar]
- 7. Ashcroft F. M. (2005) ATP-sensitive potassium channelopathies: focus on insulin secretion. J. Clin. Invest. 115, 2047–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aguilar-Bryan L., and Bryan J. (1999) Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 20, 101–135 [DOI] [PubMed] [Google Scholar]
- 9. Nichols C. G. (2006) KATP channels as molecular sensors of cellular metabolism. Nature 440, 470–476 [DOI] [PubMed] [Google Scholar]
- 10. Martin G. M., Chen P. C., Devaraneni P., and Shyng S. L. (2013) Pharmacological rescue of trafficking-impaired ATP-sensitive potassium channels. Front. Physiol. 4, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Snider K. E., Becker S., Boyajian L., Shyng S. L., MacMullen C., Hughes N., Ganapathy K., Bhatti T., Stanley C. A., and Ganguly A. (2013) Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J. Clin. Endocrinol. Metab. 98, E355–E363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P. 4th, Boyd A. E. 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., and Nelson D. A. (1995) Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268, 423–426 [DOI] [PubMed] [Google Scholar]
- 13. Yan F., Lin C. W., Weisiger E., Cartier E. A., Taschenberger G., and Shyng S. L. (2004) Sulfonylureas correct trafficking defects of ATP-sensitive potassium channels caused by mutations in the sulfonylurea receptor. J. Biol. Chem. 279, 11096–11105 [DOI] [PubMed] [Google Scholar]
- 14. Yan F. F., Casey J., and Shyng S. L. (2006) Sulfonylureas correct trafficking defects of disease-causing ATP-sensitive potassium channels by binding to the channel complex. J. Biol. Chem. 281, 33403–33413 [DOI] [PubMed] [Google Scholar]
- 15. Yan F. F., Lin Y. W., MacMullen C., Ganguly A., Stanley C. A., and Shyng S. L. (2007) Congenital hyperinsulinism associated ABCC8 mutations that cause defective trafficking of ATP-sensitive K+ channels: identification and rescue. Diabetes 56, 2339–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pratt E. B., Yan F. F., Gay J. W., Stanley C. A., and Shyng S. L. (2009) Sulfonylurea receptor 1 mutations that cause opposite insulin secretion defects with chemical chaperone exposure. J. Biol. Chem. 284, 7951–7959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen P. C., Olson E. M., Zhou Q., Kryukova Y., Sampson H. M., Thomas D. Y., and Shyng S. L. (2013) Carbamazepine as a novel small molecule corrector of trafficking-impaired ATP-sensitive potassium channels identified in congenital hyperinsulinism. J. Biol. Chem. 288, 20942–20954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou Q., Chen P. C., Devaraneni P. K., Martin G. M., Olson E. M., and Shyng S. L. (2014) Carbamazepine inhibits ATP-sensitive potassium channel activity by disrupting channel response to MgADP. Channels 8, 376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sampson H. M., Lam H., Chen P. C., Zhang D., Mottillo C., Mirza M., Qasim K., Shrier A., Shyng S. L., Hanrahan J. W., and Thomas D. Y. (2013) Compounds that correct F508del-CFTR trafficking can also correct other protein trafficking diseases: an in vitro study using cell lines. Orphanet J. Rare Dis. 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Babenko A. P., and Bryan J. (2003) SUR domains that associate with and gate KATP pores define a novel gatekeeper. J. Biol. Chem. 278, 41577–41580 [DOI] [PubMed] [Google Scholar]
- 21. Chan K. W., Zhang H., and Logothetis D. E. (2003) N-terminal transmembrane domain of the SUR controls trafficking and gating of Kir6 channel subunits. EMBO J. 22, 3833–3843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schwappach B., Zerangue N., Jan Y. N., and Jan L. Y. (2000) Molecular basis for KATP assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron 26, 155–167 [DOI] [PubMed] [Google Scholar]
- 23. Devaraneni P. K., Martin G. M., Olson E. M., Zhou Q., and Shyng S. L. (2015) Structurally distinct ligands rescue biogenesis defects of the KATP channel complex via a converging mechanism. J. Biol. Chem. 290, 7980–7991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flanagan S. E., Clauin S., Bellanné-Chantelot C., de Lonlay P., Harries L. W., Gloyn A. L., and Ellard S. (2009) Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 30, 170–180 [DOI] [PubMed] [Google Scholar]
- 25. Tornovsky S., Crane A., Cosgrove K. E., Hussain K., Lavie J., Heyman M., Nesher Y., Kuchinski N., Ben-Shushan E., Shatz O., Nahari E., Potikha T., Zangen D., Tenenbaum-Rakover Y., de Vries L., Argente J., Gracia R., Landau H., Eliakim A., Lindley K., Dunne M. J., Aguilar-Bryan L., and Glaser B. (2004) Hyperinsulinism of infancy: novel ABCC8 and KCNJ11 mutations and evidence for additional locus heterogeneity. J. Clin. Endocrinol. Metab. 89, 6224–6234 [DOI] [PubMed] [Google Scholar]
- 26. Raab-Graham K. F., Cirilo L. J., Boettcher A. A., Radeke C. M., and Vandenberg C. A. (1999) Membrane topology of the amino-terminal region of the sulfonylurea receptor. J. Biol. Chem. 274, 29122–29129 [DOI] [PubMed] [Google Scholar]
- 27. Zerangue N., Schwappach B., Jan Y. N., and Jan L. Y. (1999) A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron 22, 537–548 [DOI] [PubMed] [Google Scholar]
- 28. Fukuda Y., Aguilar-Bryan L., Vaxillaire M., Dechaume A., Wang Y., Dean M., Moitra K., Bryan J., and Schuetz J. D. (2011) Conserved intramolecular disulfide bond is critical to trafficking and fate of ATP-binding cassette (ABC) transporters ABCB6 and sulfonylurea receptor 1 (SUR1)/ABCC8. J. Biol. Chem. 286, 8481–8492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conti L. R., Radeke C. M., Shyng S. L., and Vandenberg C. A. (2001) Transmembrane topology of the sulfonylurea receptor SUR1. J. Biol. Chem. 276, 41270–41278 [DOI] [PubMed] [Google Scholar]
- 30. Hardy O. T., Hernandez-Pampaloni M., Saffer J. R., Suchi M., Ruchelli E., Zhuang H., Ganguly A., Freifelder R., Adzick N. S., Alavi A., and Stanley C. A. (2007) Diagnosis and localization of focal congenital hyperinsulinism by 18F-fluorodopa PET scan. J. Pediatr. 150, 140–145 [DOI] [PubMed] [Google Scholar]
- 31. Raphemot R., Swale D. R., Dadi P. K., Jacobson D. A., Cooper P., Wojtovich A. P., Banerjee S., Nichols C. G., and Denton J. S. (2014) Direct activation of beta-cell KATP channels with a novel xanthine derivative. Mol. Pharmacol. 85, 858–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cosgrove K. E., Straub S. G., Barnes P. D., Chapman J., Sharp G. W., and Dunne M. J. (2004) Y-26763: ATP-sensitive K+ channel activation and the inhibition of insulin release from human pancreatic beta-cells. Eur. J. Pharmacol. 486, 133–139 [DOI] [PubMed] [Google Scholar]
- 33. Nielsen F. E., Ebdrup S., Jensen A. F., Ynddal L., Bodvarsdottir T. B., Stidsen C., Worsaae A., Boonen H. C., Arkhammar P. O., Fremming T., Wahl P., Kornø H. T., and Hansen J. B. (2006) New 3-alkylamino-4H-thieno-1,2,4-thiadiazine 1,1-dioxide derivatives activate ATP-sensitive potassium channels of pancreatic beta cells. J. Med. Chem. 49, 4127–4139 [DOI] [PubMed] [Google Scholar]
- 34. Macmullen C. M., Zhou Q., Snider K. E., Tewson P. H., Becker S. A., Aziz A. R., Ganguly A., Shyng S. L., and Stanley C. A. (2011) Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the beta-cell sulfonylurea receptor SUR1. Diabetes 60, 1797–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashcroft F. M., and Gribble F. M. (1998) Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 21, 288–294 [DOI] [PubMed] [Google Scholar]
- 36. Gloyn A. L., Pearson E. R., Antcliff J. F., Proks P., Bruining G. J., Slingerland A. S., Howard N., Srinivasan S., Silva J. M., Molnes J., Edghill E. L., Frayling T. M., Temple I. K., Mackay D., Shield J. P., et al. (2004) Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. New Engl. J. Med. 350, 1838–1849 [DOI] [PubMed] [Google Scholar]
- 37. Nichols C. G., Shyng S. L., Nestorowicz A., Glaser B., Clement J. P. 4th, Gonzalez G., Aguilar-Bryan L., Permutt M. A., and Bryan J. (1996) Adenosine diphosphate as an intracellular regulator of insulin secretion. Science 272, 1785–1787 [DOI] [PubMed] [Google Scholar]
- 38. Cartier E. A., Conti L. R., Vandenberg C. A., and Shyng S. L. (2001) Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. Proc. Natl. Acad. Sci. U.S.A. 98, 2882–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taschenberger G., Mougey A., Shen S., Lester L. B., LaFranchi S., and Shyng S. L. (2002) Identification of a familial hyperinsulinism-causing mutation in the sulfonylurea receptor 1 that prevents normal trafficking and function of KATP channels. J. Biol. Chem. 277, 17139–17146 [DOI] [PubMed] [Google Scholar]
- 40. Cutting G. R. (2015) Cystic fibrosis genetics: from molecular understanding to clinical application. Nat. Rev. Genet. 16, 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nestorowicz A., Wilson B. A., Schoor K. P., Inoue H., Glaser B., Landau H., Stanley C. A., Thornton P. S., Clement J. P. 4th, Bryan J., Aguilar-Bryan L., and Permutt M. A. (1996) Mutations in the sulonylurea receptor gene are associated with familial hyperinsulinism in Ashkenazi Jews. Hum. Mol. Genet. 5, 1813–1822 [DOI] [PubMed] [Google Scholar]
- 42. Snider K. E., Becker S., Boyajian L., Shyng S. L., MacMullen C., Hughes N., Ganapathy K., Bhatti T., Stanley C. A., and Ganguly A. (2013) Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J. Clin. Endocrinol. Metab. 98, E355–E363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suchi M., MacMullen C. M., Thornton P. S., Adzick N. S., Ganguly A., Ruchelli E. D., and Stanley C. A. (2006) Molecular and immunohistochemical analyses of the focal form of congenital hyperinsulinism. Mod. Pathol. 19, 122–129 [DOI] [PubMed] [Google Scholar]
- 44. Greer R. M., Shah J., Jeske Y. W., Brown D., Walker R. M., Cowley D., Bowling F. G., Liaskou D., Harris M., Thomsett M. J., Choong C., Bell J. R., Jack M. M., and Cotterill A. M. (2007) Genotype-phenotype associations in patients with severe hyperinsulinism of infancy. Pediatr. Dev. Pathol. 10, 25–34 [DOI] [PubMed] [Google Scholar]
- 45. Otonkoski T., Näntö-Salonen K., Seppänen M., Veijola R., Huopio H., Hussain K., Tapanainen P., Eskola O., Parkkola R., Ekström K., Guiot Y., Rahier J., Laakso M., Rintala R., Nuutila P., and Minn H. (2006) Noninvasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes 55, 13–18 [PubMed] [Google Scholar]
- 46. Fernandez-Marmiesse A., Salas A., Vega A., Fernandez-Lorenzo J. R., Barreiro J., and Carracedo A. (2006) Mutation spectra of ABCC8 gene in Spanish patients with hyperinsulinism of infancy (HI). Hum. Mutat. 27, 214. [DOI] [PubMed] [Google Scholar]
- 47. De Vroede M., Bax N. M., Brusgaard K., Dunne M. J., and Groenendaal F. (2004) Laparoscopic diagnosis and cure of hyperinsulinism in two cases of focal adenomatous hyperplasia in infancy. Pediatrics 114, e520–e522 [DOI] [PubMed] [Google Scholar]
- 48. Verheul J. C., Ris-Stalpers C., Bikker H., Bakker-van Waarde W. M., and Noordam C. (2011) [Congenital hyperinsulinism in the north-east Netherlands: clinical features and DNA diagnostics in 22 children]. Ned. Tijdschr. Geneeskd. 155, A3343. [PubMed] [Google Scholar]