FIGURE 1.
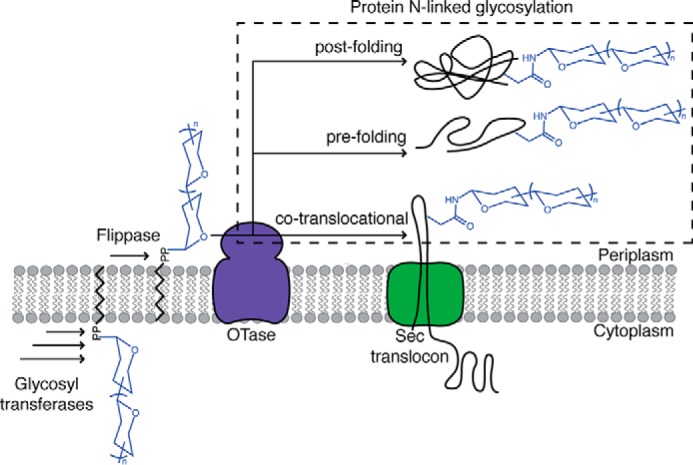
Schematic overview of bacterial N-linked glycosylation and proposed conformation states of substrate proteins during modification. Glycosyltransferases assemble a polyprenol diphosphate-linked glycan in a stepwise manner at the cytoplasmic membrane. The glycosyl donor is subsequently translocated by a flippase to the periplasmic face of the membrane. The OTase transfers the glycan onto an asparagine residue of a substrate protein. Three conformational states of substrate proteins during N-glycosylation are proposed: 1) before folding is complete, co-translocationally; 2) before folding is complete, post-translocationally; 3) after folding is complete, post-translocationally.
