FIGURE 10.
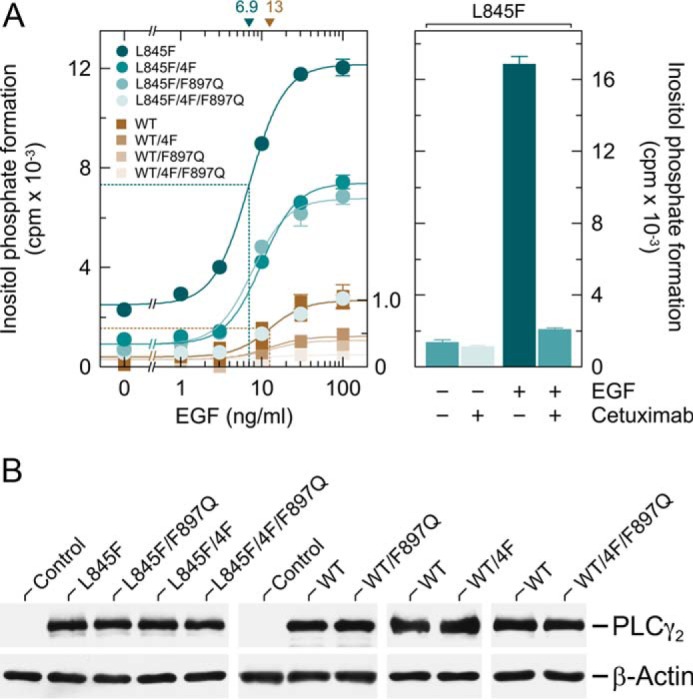
PLCγ2L845F is hypersensitive to activation by EGF receptor(s) endogenously expressed in COS-7 cells by a mechanism dependent on both protein tyrosine phosphorylation and activation by Rac. A, COS-7 cells were transfected with 150 ng/well vectors encoding either PLCγ2L845F (L845F), PLCγ2L845F/4F (L845F/4F), PLCγ2L845F/F897Q (L845F/F897Q), or PLCγ2L845F/4F/F897Q (L845F/4F/F897Q) or wild-type PLCγ2 (WT), PLCγ24F (WT/4F), PLCγ2F897Q (WT/F897Q), or PLCγ24F/F897Q (WT/4F/F897Q) (all constructed in pMT2 vector; Tyr → Phe substitutions at amino acid positions 753, 759, 1197, and 1217). Eighteen hours after transfection, the cells were incubated for a further 24 h with myo-[2-3H]inositol in the absence of serum and then treated for 60 min in the presence of 20 mm LiCl with increasing concentrations of EGF (1, 3, 10, 30, and 100 ng/ml), followed by determination of inositol phosphate formation. Background inositol phosphate formation in response to addition of EGF at increasing concentrations was determined in parallel on cells transfected with empty vector and subtracted from the individual values, with appropriate consideration of error propagation (68). The data on PLCγ2L845F and its mutants are from one experiment; the data on wild-type PLCγ2 and its mutants are from three experiments, each comparing the activity of wild-type PLCγ2 and one of the mutants. The latter activities were normalized to the maximal activity of PLCγ2L845F/4F/F897Q used as an internal control in one of the latter experiments (shown as a fraction of 1.0 on the right y axis). The EC50 values of EGF for the stimulation of wild-type or L845F mutant PLCγ2 activity obtained by non-linear curve fitting are shown above the graphs in nanograms/ml (left panel). In the right panel, COS-7 cells were transfected with 150 ng/well vector encoding PLCγ2L845F (L845F) and then incubated as described above and treated for 60 min in the absence or presence of 20 μg/ml cetuximab without or with 10 ng/ml EGF in medium containing 20 mm LiCl prior to determination of inositol phosphate formation. B, homogenates from cells functionally analyzed in A, left panel, were subjected to SDS-PAGE and immunoblotting using an antiserum reactive against PLCγ2 or antibody reactive against β-actin.
