FIGURE 6.
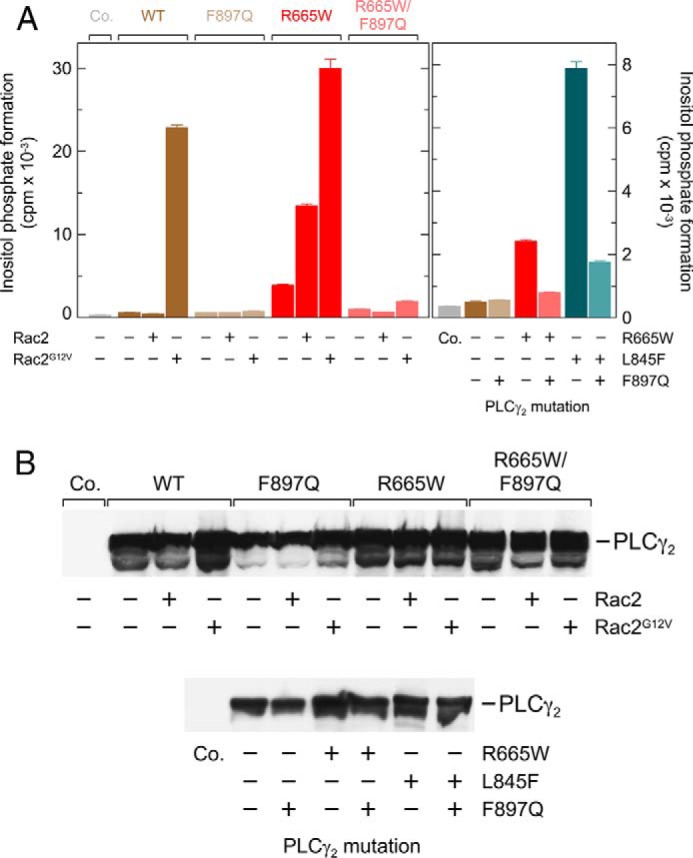
Enhanced Rac2- and Rac2G12V-stimulated activity of PLCγ2R665W as well as enhanced basal activity of PLCγ2R665W and PLCγ2L845F are prevented by a point mutation of PLCγ2, F897Q, mediating resistance of the enzyme to stimulation by activated Rac2. A, COS-7 cells were transfected with 500 ng/well empty vector (Co., Control) or vector encoding either wild-type PLCγ2 (WT), PLCγ2F897Q (F897Q), PLCγ2R665W (R665W), or PLCγ2 R665W/F897Q (R665W|F897Q), together with 25 ng/well empty vector or vector encoding Rac2 or Rac2G12V (left panel). In the right panel, COS-7 cells were transfected with 500 ng/well (from left to right) empty vector (Co.) or vector encoding either wild-type PLCγ2, PLCγ2F897Q, PLCγ2R665W, PLCγ2 R665W/F897Q, PLCγ2L845F, or PLCγ2 L845F/F897Q. Note that the vectors encoding mutant PLCγ2 were used at the same maximal amount (500 ng/well) to observe the stimulation by wild-type Rac2 (left panel) and enhanced basal activity of the PLCγ2 mutants (right panel).Twenty four hours after transfection, the cells were incubated for 20 h with myo-[2-3H] inositol, and inositol phosphate formation was determined. B, homogenates from cells functionally analyzed in A were subjected to SDS-PAGE and immunoblotting using an antibody reactive against the c-Myc epitope.
