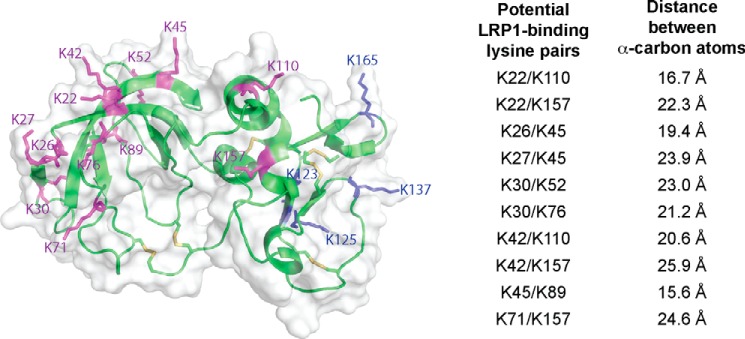
FIGURE 1.
Identification of potential LRP1-binding residues in TIMP-3. A model of TIMP-3 was generated using the available crystal structure of TIMP-2. The position of Lys-180 was unresolved in the model, but the remaining 16 lysine residues of TIMP-3 were all predicted to be located on the surface of the molecule. Lysine residues located on the N-terminal domain of TIMP-3 are indicated in purple, and those on the C-terminal domain are shown in blue. The distance between α-carbon residues of pairs of lysine residues was measured, and 10 pairs of lysine residues (listed on the right) were predicted to be separated by 21 ± 5 Å. This figure was made with PyMOL.