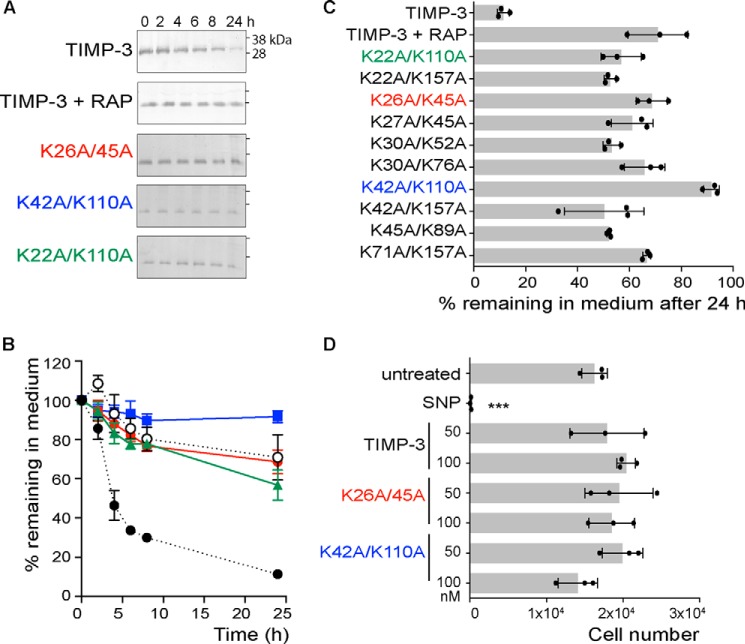
FIGURE 3.
Mutation of pairs of lysine residues further increases the half-life of TIMP-3 in the medium of HTB94 chondrosarcoma cells. A, HTB94 chondrosarcoma cells were incubated (0–24 h) with 1 nm FLAG-tagged wild-type TIMP-3, TIMP-3 preincubated with 1 μM RAP, TIMP-3 K26A/K45A, TIMP-3 K42A/K110A, or TIMP-3 K22A/K110A in DMEM with 0.1% FCS. Media were concentrated by TCA precipitation and analyzed by immunoblotting with anti-FLAG M2 antibody. The loss of each mutant from the medium was calculated relative to its pixel volume at t = 0 h (defined as 100%). B, data were analyzed by densitometry using Phoretix 1D software (mean ± S.D. (error bars), n = 3 technical replicates). Levels of TIMP-3 (black circle), TIMP-3 preincubated with 1 μM RAP (white circle), and the TIMP-3 mutants K26A/K45A (red circle), K42A/K110A (blue square), and K22A/K110A (green triangle) in the medium are shown. C, the percentage of each TIMP-3 double mutant (mean ± S.D., n = 3 technical replicates) remaining in the medium at 24 h was calculated. After 24 h, all mutants were present in the medium at significantly (p ≤ 0.001) higher levels than wild-type TIMP-3, with K26A/K45A or K42A/K110A remaining in the medium at the highest concentration. D, HTB94 chondrosarcoma cells were treated with purified wild-type TIMP-3, K26A/K45A, or K42A/K110A (50 or 100 nm) or sodium nitroprusside (SNP; 10 mm) for 24 h, and cell viability (mean ± S.D., n = 3 technical replicates) was assessed using the MTS assay. Sodium nitroprusside caused significant cell toxicity (***, p < 0.001), whereas wild-type and mutant TIMP-3 had no effect on cell viability at the concentrations tested (p > 0.05).