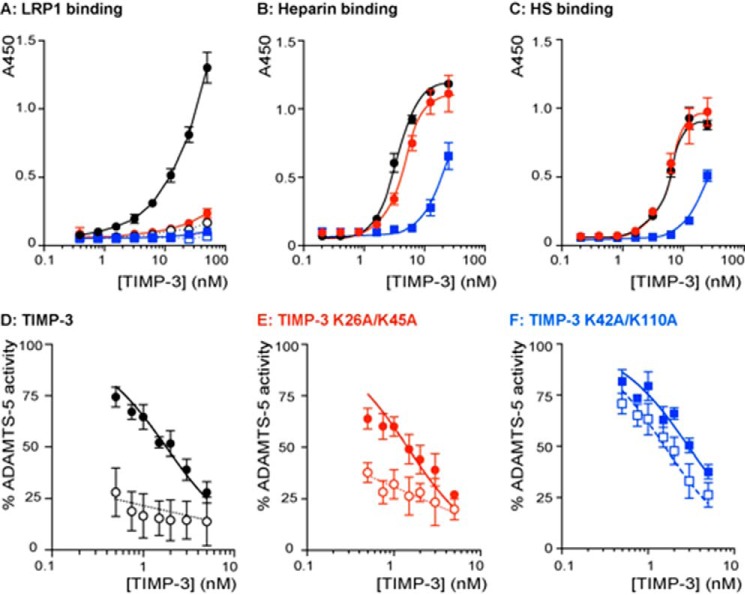
FIGURE 6.
Characterization of TIMP-3 K26A/K45A and K42A/K110A binding properties. A, binding of wild-type TIMP-3 (black circle), K26A/K45A (red circle), and K42A/K110A (blue square) to LRP1 was analyzed by ELISA. Medium-binding ELISA plates were coated with LRP1 (5 nm) overnight and incubated with 0.4–50 nm wild-type or mutant TIMP-3, either alone (closed symbols) or preincubated with heparin (open symbols; 150 μg/ml, 1 h, 37 °C). Bound protein was detected using an M2 anti-FLAG primary antibody and anti-mouse-HRP secondary antibody. Hydrolysis of 3,3′,5,5′-tetramethylbenzidine substrate was analyzed by measuring absorbance at 450 nm (mean ± S.D. (error bars), n = 3 technical replicates). B, binding of wild-type TIMP-3 (black circle), K26A/K45A (red circle), and K42A/K110A (blue square) to heparin was analyzed by ELISA. Glycosaminoglycan-binding ELISA plates were coated with heparin (10 μg/ml) overnight and incubated with 0.4–25 nm wild-type or mutant TIMP-3. Bound protein was detected as in A (mean ± S.D., n = 3 technical replicates). C, binding of wild-type TIMP-3 (black circle), K26A/K45A (red circle), and K42A/K110A (blue square) to heparan sulfate was analyzed by ELISA. Glycosaminoglycan-binding ELISA plates were coated with heparan sulfate (10 μg/ml) overnight and incubated with 0.4–25 nm wild-type or mutant TIMP-3. Bound protein was detected as in A (mean ± S.D., n = 3 technical replicates). D, ADAMTS-5 (0.5 nm) was incubated with wild-type TIMP-3 (0.5–10 nm) without (black circle) or with heparin (100 nm, white circle) for 1 h at 37 °C, and residual enzyme activity was quantified (18 h, 37 °C, mean ± S.D., n = 3 technical replicates). E, ADAMTS-5 (0.5 nm) was incubated with K26A/K45A (0.5–10 nm) without (black circle) or with heparin (100 nm, white circle) for 1 h at 37 °C, and residual enzyme activity was quantified (18 h, 37 °C, mean ± S.D., n = 3 technical replicates). F, ADAMTS-5 (0.5 nm) was incubated with K42A/K110A (0.5–10 nm) without (blue square) or with heparin (100 nm, white square) for 1 h at 37 °C, and residual enzyme activity was quantified (18 h, 37 °C, mean ± S.D., n = 3 technical replicates).