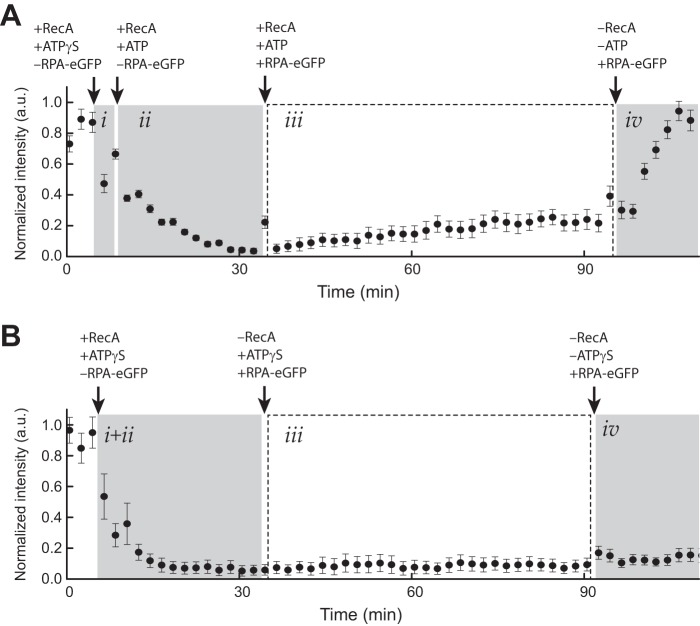
FIGURE 2.
RecA presynaptic complex stability. A, graphical depiction of RPA-eGFP signal intensity (a.u.) averaged over 11 ssDNA molecules during assembly of the RecA presynaptic filament in the presence of ATP. Panel i corresponds to the brief nucleation phase conducted in the presence of 1 mm ATPγS and the absence of free RPA-eGFP. Panel ii shows nucleation was followed by filament elongation with 1 mm ATP, 50 nm RecA in the absence of free RPA-eGFP. In panel iii, the sample chamber was then flushed with buffer containing 1 mm ATP, 50 nm free RecA, and 0.2 nm free RPA-eGFP, which was used to monitor RecA dissociation from the ssDNA. Panel iv, finally, the sample chamber was flushed with buffer with no ATP and no free RecA causing the bound RecA to dissociate from the ssDNA, which is revealed as the reappearance of the RPA-eGFP signal. The error bars represent standard deviation measured from 11 individual ssDNA molecules. B, graphical depiction of RPA-eGFP signal intensity (a.u.) averaged over 11 ssDNA molecules during assembly of the RecA presynaptic filament in the presence of only ATPγS. Reactions were performed as in A with the exception that 1 mm ATPγS was substituted for ATP in all stages of the reaction.