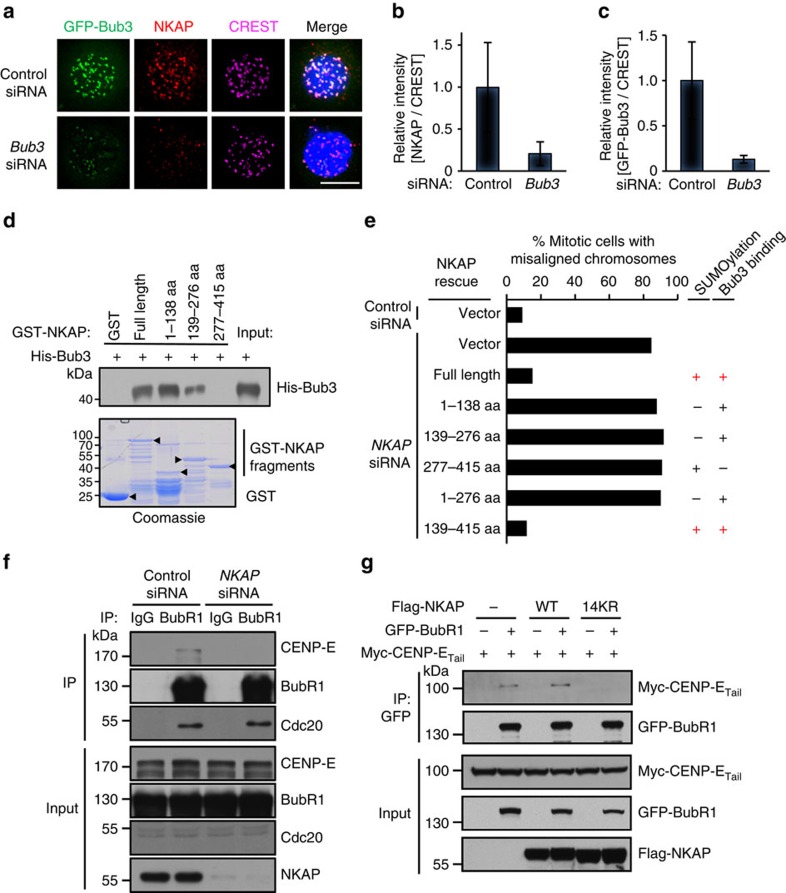
Figure 6. Bub3 recruits NKAP to stabilize BubR1-CENP-E interaction at kinetochores.
(a) Bub3 knockdown impairs NKAP kinetochore localization. HeLa/GFP-Bub3 cells were treated with control or Bub3 siRNA for 48 h and synchronized in prometaphase with nocodazole (100 ng ml−1) and MG132 (10 μM) for 4 h. The cells were stained with anti-NKAP antibody (red), kinetochores (CREST, magenta) and DNA (blue). Scale bar, 10 μm. (b) Quantification of relative fluorescence intensity of the NKAP signal on kinetochores in cells as described in a. Fluorescence intensity of NKAP in control cells was normalized to 1 (n≥10 cells, >200 kinetochores for each group). Cells were randomly selected for observation and statistics. Data are shown as mean±s.d. (c) Knockdown efficiency of siRNA targeting Bub3. (d) NKAP directly binds Bub3. Recombinant His-Bub3 protein was incubated with GST, GST-NKAP and its deletion mutants. The bound proteins were determined by immunoblot. The amounts of recombinant proteins from the same reaction were analysed by SDS–PAGE and Coomassie blue staining. Arrowheads indicate the positions of corresponding proteins. (e) Deletion analysis of NKAP regions responsible for chromosome alignment. HeLa/GFP-H2B cells were transfected with siRNA-resistant RFP-NKAP constructs for 6 h, preceded by transfection with NKAP siRNA for 42 h. The percentage of cells with misaligned chromosomes is quantified for cells expressing each construct. The SUMOylation and Bub3-binding ability of each construct are shown. More than 200 cells were counted in each group. Data are representative of two independent experiments. (f) The endogenous association of CENP-E with BubR1 is decreased in NKAP-knockdown cells. HeLa cells were transfected with control or NKAP siRNA and synchronized at prometaphase with thymidine–nocodazole treatment. IP was carried out using anti-BubR1 antibody and the IPs were probed with indicated antibodies. (g) Wild type NKAP stabilizes the interaction of BubR1 and CENP-E whereas SUMO-null mutant impairs their interaction. GFP-BubR1 and Myc-CENP-ETail were transfected into HEK293T cells, together with Flag-NKAP or its 14KR mutant. Cells were synchronized in mitosis with thymidine–nocodazole treatment and shaken-off. GFP-BubR1 was immunoprecipitated using anti-GFP resin and probed with indicated antibodies.