Table 1.
RCO-, melting points (m.p.), and yields of N-acylbenzotriazoles 3a-k′ and N-acylcephalexines 4a-k′.
| RCO-a–k′ | 3a–k′ Yield % | 3a–k′m.p. (°C) m.p. (°C) [Lit.] | Time/h 4a–k′ | 4a–k′ Yield % | 4a–k′ m.p. (°C) |
|---|---|---|---|---|---|
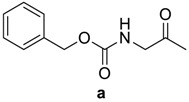 |
94 | 107–109 (106–108) [24] | 10 | 87 | 210–212 |
 |
95 | 150–153 (151–152) [24] | 12 | 86 | 216–218 |
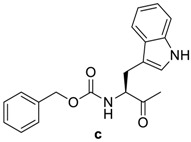 |
95 | 99–101 (100–101) [25] | 12 | 84 | 168–170 |
 |
94 | 111–112 (112–113) [14] | 9 | 84 | 132–134 |
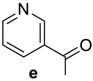 |
94 | 101–102 (101–102) [15] | 8 | 82 | 200–202 |
 |
90 | 156–157 (155–156) [16] | 10 | 87 | 226–228 |
 |
96 | 125–127 (126–128) [17] | 6 | 91 | 197–199 |
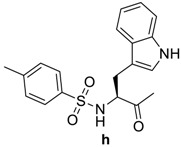 |
87 | 175–176 | 12 | 86 | 125–127 |
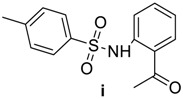 |
89 | 148–150 | 12 | 82 | 137–139 |
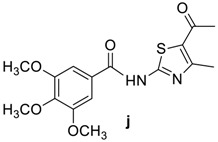 |
82 | 107–110 | 20 | 89 | 179–181 |
 |
90 | 142–144 | 14 | 93 | 234–236 |
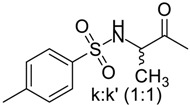 |
90 | 142–144 | 14 | 89 | 224–227 |
