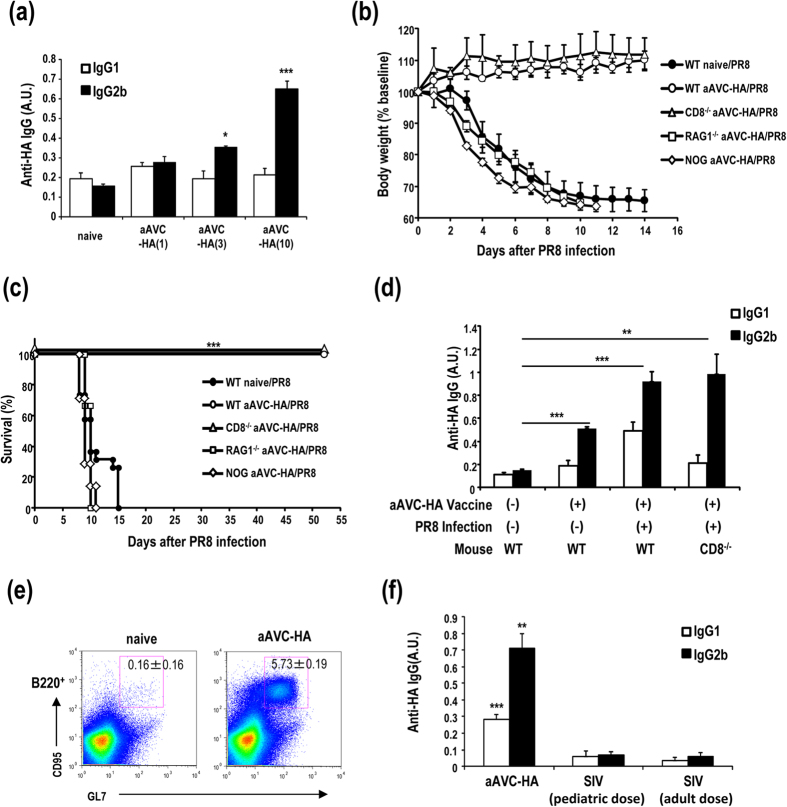
Figure 8. Vaccination with aAVC-HA protects against influenza virus infection.
(a) HA-specific serum IgG1 and IgG2b antibody response were measured in WT mice 2 weeks after immunization with aAVC-HA (1, 3, or 10 μg HA mRNA-transfected aAVC-HA). (Mean ± SEM, n = 4) *P < 0.05, ***P < 0.001. (b,c) WT, CD8−/−, Rag1−/− or NOG mice were vaccinated with 5 × 105 aAVC-HA, and then challenged with lethal dose of PR8 influenza virus 2 weeks later. These mice were monitored for weight loss (b) and survival (c). WT mice without aAVC-HA vaccination and challenged with PR8 were examined as a control. (Mean ± SEM, n = 7–11) ***P < 0.001 (d) Serum anti-HA-specific antibody isotype was assessed by ELISA at day14 after aAVC-HA immunization or day 28 after aAVC-HA immunization, and subsequently PR8 infection. (Mean ± SEM, n = 4–7) **P < 0.01, ***P < 0.001 (e) GC formation was examined by flow cytometry analysis using B220-APC, CD95-PE, and GL-7-FITC. (Mean ± SEM, n = 4) (f) Comparison of the HA-specific antibody response to vaccination with aAVC-HA or the standard influenza vaccine (SIV). For these studies, the two standard doses of standard influenza vaccine (0.75 μg/kg for human pediatric dose and 0.3 μg/kg for human adult dose) or the aAVC-HA were administered to C57BL/6 mice. Serum HA-specific antibody was assessed by ELISA at day14 after vaccination. (Mean ± SEM, n = 4–5) **P < 0.01, ***P < 0.001.