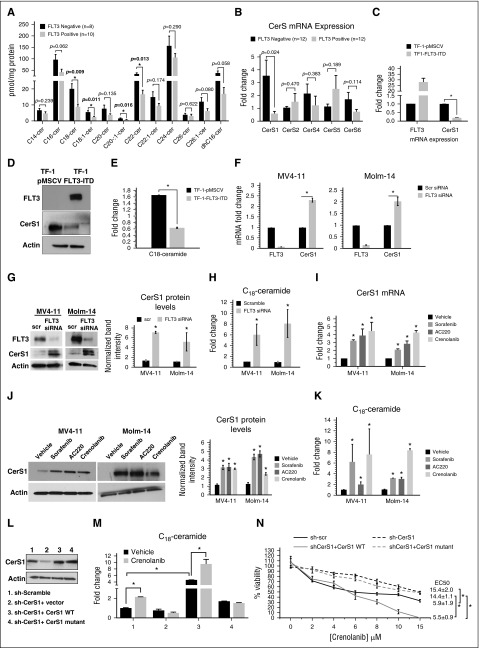
Figure 1.
Reactivation of CerS1-C18-ceramide axis is required for the response of AML cells to FLT3-targeted therapy. (A) MS high-performance liquid chromatography (HPLC)-MS-MS analysis for the different ceramide species in FLT3+ vs FLT3− CD34+ AML blasts obtained from bone marrow of AML patients. (B) Quantitative polymerase chain reaction (qPCR) measurement of CerSs mRNA expression in FLT3+ vs FLT3− CD34+ AML blasts obtained from bone marrow of AML patients. (C) CerS1 mRNA in TF-1 cells transfected with FLT3-ITD overexpression vector. (D) Western blot for CerS1 protein in TF-1 cells transfected with FLT3-ITD overexpression vector. (E) HPLC-MS-MS measurement of C18-ceramide in TF-1 cells transfected with FLT3-ITD overexpression vector. (F) qPCR measuring CerS1 mRNA in cells transfected with FLT3 small interfering RNA (siRNA). (G) qPCR measuring CerS1 mRNA in cells treated with FLT3 pharmacological inhibitors. (H) Western blotting measuring CerS1 protein in cells transfected with FLT3 siRNA. (I) Western blotting measuring CerS1 protein in cells treated with FLT3 pharmacological inhibitors. (J) HPLC-MS-MS measuring C18-ceramide in cells transfected with FLT3 siRNA. (K) HPLC-MS-MS measuring C18-ceramide in cells treated with FLT3 pharmacological inhibitors. (L) Western blot to detect CerS1 protein in sh-CerS1 cells reconstituted with CerS1 WT or CerS1-H138A catalytically inactive mutant. (M) HPLC-MS-MS measurement of C18-cermide in crenolanib-treated cells transfected with CerS1 WT or CerS1-H138A catalytically inactive mutant. (N) Percentage of viability measured using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-dimethyltetrazolium bromide) assay for sh-scr, sh-CerS1, sh-CerS1+CerS1 WT, and sh-CerS1+CerS1 H1338A mutant, treated with crenolanib. Values indicate mean ± standard deviation (SD) of n = 3 independent experiments. *P value of <.05 using the 2-sided Student t test.