Figure 1.
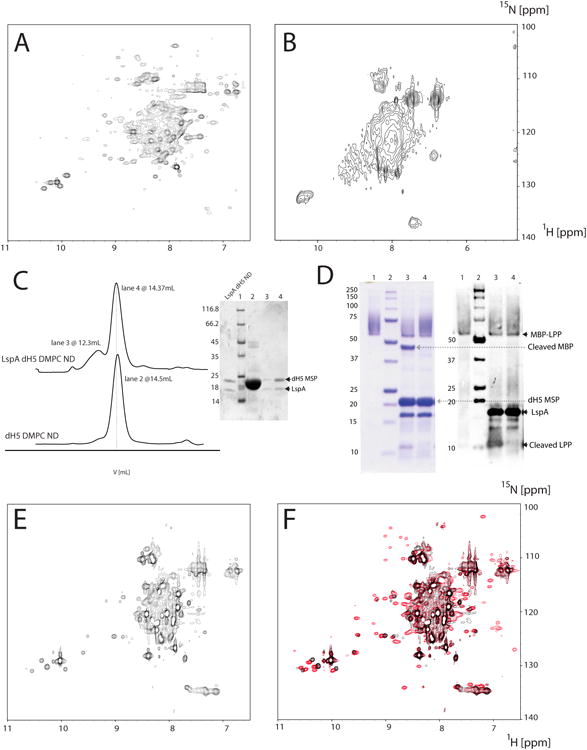
Sample optimization and characterization of LspA. 15N-labeled LspA in DDM from E.coli (A) and 2H/15N-labeled LspA in DPC from P-CF expression (B). (C) Size exclusion chromatograms of empty and LspA dH5 DMPC NDs. The molecular weight obtained by calibration of an analytical Superdex 200 is 95kDa for empty dH5 DMPC NDs and 102kDa for LspA embedded dH5 DMPC NDs. Buffer conditions were 20mM TrisHCl pH 8, 100mM NaCl. The majority of LspA in dH5 DMPC NDs are in a single homogenous peak (lane 4 of the Coomassie stained gel) that is slightly larger than empty dH5 DMPC NDs (lane 2). A small amount of LspA is also present in larger aggregates (lane 3). (D) Functional assay of LspA in dH5 DMPC NDs. Active LspA (lane 3) cleaves at the lipobox of the substrate fusion protein MBP-LPP (lane 1) and free MBP and free LPP are visible on SDS-PAGE gel and the western blot respectively. Lane 4 contains LspA in dH5 DMPC ND incubated with globomycin and does not cleave MBP-LPP. Free MBP and dH5 MSP are absent in lanes 3 and 4 of the western blot due to their consistently poor visualization with the anti-his antibody. (E) [15N-1H] TROSY of LspA in dH5 DMPC ND without globomycin. (F) Overlay of a [15N-1H] TROSY of LspA in dH5 DMPC NDs with globomycin (red) and the spectrum shown in (E) without the inhibitor.
