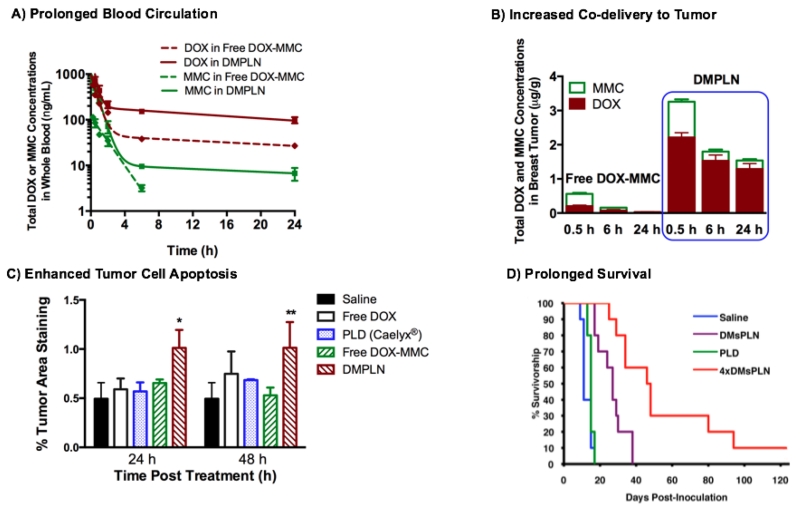
Figure 6.
A) Whole blood analysis of pharmacokinetics of DOX and MMC co-delivered by DMPLN or in free drug combination; B) Breast tumor accumulation of DOX and MMC co-delivered by DMPLN or in free drug combination; C) Breast tumor cell apoptosis determined by percent activation of caspase-3 treated by DMPLN and other DOX formulations; D) Kaplan-Meier survival curves of breast tumor bearing mice treated with DMPLN (1× or 4× injections) or clinically used PLD (Caelyx®) (1× injection). An orthotopic EMT6/WT breast tumor model was used and treated with i.v. administration of various DOX and DOX-MMC formulations at equivalent drug doses (9.2 mg/kg DOX or in additional 2.9 mg/kg MMC). The figures are permitted and reproduced from Zhang et al. 2016 [55] and Shuhendler et al. 2014 [54].