Abstract
Background
Head and neck cancer in Indian perspective predominantly relates to tobacco use. The present study explores the prevalence of oral ulcers and its association with addictions among the population of Uttar Pradesh and Rajasthan, India.
Methodology
The screening method in early detection of head and neck cancer is broadly symptom based. 1399 subjects from Uttar Pradesh and Rajasthan were screened by trained personnel between April and June 2015.
Results
Study findings showed, mouth ulcers and trismus were common symptoms and tobacco chewing and smoking were common addictions. There were statistically significant associations among the symptoms and addictions as well as predominance in rural populations. The majority of smokers (27.1%) belonged to age ≥55 years whereas the tobacco chewers (29.2%) and alcohol abusers (45.8%) in the age group 25–34 years. Also the risk of developing mouth ulcers and trismus in this area are approximately 35 (MRR: 35.7, 95% CI: 15.5–81.9) and nearly eight (MRR: 7.7, 95% CI: 2.2–26.6) times higher respectively in males. However, joint use of smoked and smokeless tobacco increases nearly three times more risk of either mouth ulcers or trismus.
Conclusion
Male individuals are more exposed to certain addictions such as tobacco (smoked and smokeless) and alcohol. The prevalence of oral ulcers is primarily associated with the addictions. Therefore, these persons are more at risk of further developing head neck cancer. A large level community screening and awareness are required especially among the rural population of India.
Keywords: Oral ulcers, Community based visual screening, Tobacco (smoked and smokeless), Precancerous lesions, Head neck cancer
1. Introduction
Oral cancer is the commonest cancer topping the cancer registries in India. The disease load and the symptom burdens are of particular importance in head and neck cancer patients in India. In South-central Asia, the oral cavity and oropharynx are commonest subsites, where 80% of head and neck cancers are found.1 In India, about 200,000 new cases of head neck cancer are detected in every year.2 There are several premalignant oral mucosal lesions including leukoplakia, erythroplakia, lichen planus as well as oral submucous fibrosis (OSMF), and all carry an increased risk for malignant transformations in the oral cavity and it is associated with areca nuts and tobacco use.3 The other causes for oral mucosal lesions could be due to infection (bacterial, viral, fungal), local trauma and or irritation (traumatic keratosis, irritational fibroma, burns), systemic disease (metabolic or immunological), or related to lifestyle factors such as the usage of betel quid or alcohol.4
Screening and early detection are very useful methodologies, since precancerous lesions, in situ carcinoma as well as early stage of head and neck cancer have significantly good survival outcome after treatment.5, 6 The screening method of oral cavity malignancies is relatively simple and can be done effectively by visual inspections.7 It is also mentioned in the literature that visual inspection of oral cavity by proper trained personnel is well accepted and accurate method of screening for oral cavity malignancies.8, 9, 10 Oral self examination is a simple way of assessing self-perception of oral health and its validity has been proven. It is also a cost effective, less time consuming procedure.11
Literature mentions that visual inspection method for oral screening could be restricted to high-risk individuals and organized visual screening is a worthwhile initiative of control for oral cancer in addition to primary prevention efforts to reduce tobacco and alcohol use,12 as well as, it has potential to prevent deaths due to oral cancer.13 Despite the fact, that the oral cavity is accessible for visual examination and those oral cancers and premalignant lesions have well-defined clinical diagnostic features, oral cancers are typically detected in their advanced stages in our country. In fact, in India, 60–80% of patients present with advanced disease, as compared to 40% in developed countries.14 Consistent with patients presenting for medical care with more advanced disease in India compared with developed countries, overall survival is also reduced.15, 16 Early detection would not only improve the cure rate, but it would also lower the cost and morbidity associated with treatment. For planning of national or regional oral health promotion programs, as well as to prevent and treat oral health problems, baseline data about the magnitude of the problem is required. India has a vast geographic area, divided into states, which differ with regard to their socioeconomic, educational, cultural, and behavioral traditions.17, 18
There were no such organized screening programs undertaken till date in the western parts of Uttar Pradesh and eastern Rajasthan in a larger scale, to find out the disease burden of oral ulcers and its major associating factors. Therefore the present study primarily focuses on the distribution of oral ulcers and its association with addictions among the rural population of the region and the effectiveness of the questionnaire designed in house to collect baseline data. The other objectives were to sensitize health professionals, train community for oral self examination and to generate baseline data for previously rural population of Western Uttar Pradesh and eastern Rajasthan.
2. Methods
2.1. Data collection
Numbers of screening camps were organized in the western Uttar Pradesh and eastern Rajasthan including; Hatras, Bharatpur, Deeg, Tundla, Shikohadabad, Jhagina, Firozabad, Mathura, Shergarh, Jait and Barsana between April 2015 and June 2015. The study participants were screened by trained health-workers under the supervision of medical graduates. The outreach team collected information on demographic, symptoms and various addiction patterns among male and female subjects of all age groups. The common symptoms of head neck cancers – including ulcers in oral cavity, difficulties in opening mouth, hoarseness of voice, neck swellings, difficulty in swallowing, earache/ear discharge or nose bleeding were included in the questionnaire. Details of different areas of inspection of oral cavity, oropharynx, ear, and nose as well as the different palpation areas for neck nodes and thyroid swellings were included in the same questionnaire.
2.2. Training of health professionals
A series of training schedules were conducted by clinical oncologists, to teach the outreach group about organized history taking, to find out the important positive and negative points in history, addiction history and other relevant points. They all were taught by clinical oncologists about the details of examination of oral cavity by visual inspection, as well as palpation methods. The hands-on training was mainly focused on to undertake oral visual inspection, identify lesions suggestive of being precancerous in the oral cavity (e.g. homogeneous leucoplakia, non-homogeneous leucoplakia, erythroplakia, oral submucous fibrosis), and identify oral cancer. Two manuals on visual inspection with color photographs and descriptions of oral ulcers were used for training and reference during screening.14, 15 The competency of the all team members was examined by the clinical oncologists after successful completion of the training program.
A pilot study was conducted to pre-test the questionnaire in selected study areas of Western Uttar Pradesh prior to conducting the screening camps. The results of the pre-testing provided useful information in improving the clarity of questions for finalization of the questionnaire. The internal consistency of the questionnaire was estimated to 78% using Cronbach's alpha that indicated good level of reliability.
2.3. Statistical analysis
Data collected from the screened individuals were analyzed to estimate the prevalence of addictions and its association with the increasing risk of symptoms. Student's t test was used to compare any significance in age distribution between male and female. Chi-square or Fisher's test was applied to examine the association between two categorical variables such as age groups, gender and symptoms with type of addictions.19 Multivariable logistic regression analysis was used to estimate the odds ratios and named as multivariate rate ratios (MRR) with respect to the reference category for comparison and identification of factors associated with a higher risk of developing symptoms.20 The data were analyzed using IBM SPSS statistics version 21.0 (Armonk, NY, USA). p < 0.05 was considered for statistical significance.
2.4. Ethical considerations
The individual verbal consents were taken prior to screening of oral ulcers and making observations on addictions of tobacco use (smoked or smokeless), alcohol and betel nut. This was in line with the documentation with ethics committee.
3. Results
A cohort of 1399 persons of all age groups ranging from 12 to 86 years, who came for their screening of oral cavity in the general camp under the outreach programme run by Nayati Healthcare were considered. Their addiction habits and other epidemiological details were also taken into consideration.
The background characteristics of the screened individuals are presented in Table 1. The mean age of participants was 41.7 ± 14.6 years and 51.2% (702/1399) of them were female. Age was homogeneously distributed and there were no statistical significant difference (p > 0.05) between male (41.7 ± 15.7 years) and female (41.7 ± 13.5 years). The primary symptoms were ulcer in mouth (11.9%) followed by difficulty in opening mouth (2.6%), hoarseness (1.4%), swallowing difficulty and ear discharge at 0.8% respectively. The common addiction was tobacco chewing (11.4%) followed by smoking (8.8%) and alcohol (1.7%) respectively.
Table 1.
Background demographic and clinical profile the of study participants.
| Sample characteristics | n | % |
|---|---|---|
| Total individuals screened | 1399 | 100.0 |
| Mean age (years) (mean ± SD) | 41.7 ± 14.6 | |
| Age range | 12–86 years | |
| Gender | ||
| Male | 697 | 49.8 |
| Female | 702 | 51.2 |
| Symptoms | ||
| Mouth ulcers | 167 | 11.9 |
| Dysphasia | 11 | 0.8 |
| Nose bleeding | 5 | 0.4 |
| Ear discharge | 11 | 0.8 |
| Neck swelling | 7 | 0.5 |
| Hoarseness of voice | 19 | 1.4 |
| Trismus | 36 | 2.6 |
| Restricted tongue movement | 1 | 0.1 |
| Addiction habits | ||
| Tobacco smoking | 123 | 8.8 |
| Tobacco chewing | 159 | 11.4 |
| Alcohol consumption | 24 | 1.7 |
| Betel nut use | 2 | 0.2 |
The prevalence of different symptoms and its association with various addictions are presented in Table 2. Prevalence of mouth ulcers (17.4% among smokers and 22.8% among tobacco chewers) was significantly related with smokers (p < 0.01) and tobacco chewers (p < 0.01) respectively; dysphagia (18.2% among smokers, 27.3% among tobacco chewers and 9.1% among alcohol users) was significant with all addictions except for betel nut since it did not have sufficient numbers for statistical comparison. Prevalence of ear discharge (18.2% among smokers) and hoarseness (21.1% among smokers) was significant with smoking habit only. Proportion of trismus (25% among smokers and 44.4% among tobacco chewers) has been showing significant association (p < 0.01) with the smokers and tobacco chewers, more prominent in the tobacco chewer group. Further, the overall prevalence of tobacco smoking, tobacco chewing, alcohol abuse and betel nut chewing was significantly distributed (p < 0.05). Pattern of addiction habits were almost similar across the age groups (p > 0.05). The prevalence of smoking (16.9%) and tobacco chewing (22.1%) was higher among males and is statistically associated (p < 0.001), when compared to the addiction pattern in females.
Table 2.
Differences in prevalence and pattern of addiction across demographic and different symptom characteristics.
| Background characteristics | N | Types of addiction |
Any addictiona |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoking |
Tobacco chewing |
Alcohol |
Betel nut |
n | % | ||||||
| n | % | n | % | n | % | n | % | ||||
| Age group (years) | |||||||||||
| <25 | 159 | 12 | 7.5 | 16 | 10.1 | 2 | 1.3 | – | – | 22 | 13.8 |
| 25–34 | 299 | 28 | 9.4 | 47 | 15.7 | 11 | 3.7 | 2 | 0.7 | 64 | 21.4 |
| 35–44 | 346 | 25 | 7.2 | 33 | 9.5 | 7 | 2.0 | – | – | 40 | 11.6 |
| 45–54 | 279 | 23 | 8.2 | 31 | 11.1 | 2 | 0.7 | – | – | 42 | 15.1 |
| ≥55 | 316 | 35 | 11.1 | 32 | 10.1 | 2 | 0.6 | – | – | 58 | 18.4 |
| p value | |||||||||||
| Gender | |||||||||||
| Male | 697 | 118 | 16.9 | 154 | 22.1 | 24 | 3.4 | 2 | 0.3 | 217 | 31.1 |
| Female | 702 | 5 | 0.7 | 5 | 0.7 | – | – | – | – | 9 | 1.3 |
| p value | p < 0.001 | p < 0.001 | – | – | – | p < 0.001 | |||||
| Mouth ulcers | |||||||||||
| Absent | 1232 | 94 | 7.6 | 121 | 9.8 | 21 | 1.7 | 2 | 0.2 | 178 | 14.4 |
| Present | 167 | 29 | 17.4 | 38 | 22.8 | 3 | 1.8 | – | – | 48 | 28.7 |
| p value | p < 0.01 | p < 0.01 | p > 0.05 | – | – | p < 0.05 | |||||
| Dysphasia | |||||||||||
| Absent | 1388 | 121 | 8.7 | 156 | 11.2 | 23 | 1.7 | 2 | 0.1 | 222 | 16.0 |
| Present | 11 | 2 | 18.2 | 3 | 27.3 | 1 | 9.1 | – | – | 4 | 36.4 |
| p value | p < 0.05 | p < 0.01 | p < 0.05 | – | – | p < 0.05 | |||||
| Nose bleeding | |||||||||||
| Absent | 1394 | 123 | 8.8 | 159 | 11.4 | 24 | 1.7 | 2 | 0.1 | 226 | 16.2 |
| Present | 5 | – | – | – | – | – | – | – | – | – | – |
| p value | – | – | – | – | – | ||||||
| Ear discharge | |||||||||||
| Absent | 1388 | 121 | 8.7 | 158 | 11.4 | 24 | 1.7 | 2 | 0.1 | 224 | 16.1 |
| Present | 11 | 2 | 18.2 | 1 | 9.1 | – | – | – | – | 2 | 18.2 |
| p value | p < 0.05 | p > 0.05 | – | – | p > 0.05 | ||||||
| Neck swelling | |||||||||||
| Absent | 1392 | 122 | 8.8 | 159 | 11.4 | 24 | 1.7 | 2 | 0.1 | 225 | 16.2 |
| Present | 7 | 1 | 14.3 | – | – | – | – | – | – | 1 | 14.3 |
| p value | p > 0.05 | – | – | – | p > 0.05 | ||||||
| Hoarseness of voice | |||||||||||
| Absent | 1380 | 119 | 8.6 | 158 | 11.4 | 24 | 1.7 | 2 | 0.1 | 222 | 16.1 |
| Present | 19 | 4 | 21.1 | 1 | 5.3 | – | – | – | – | 4 | 21.1 |
| p value | p < 0.01 | p > 0.05 | – | – | p > 0.05 | ||||||
| Trismus | |||||||||||
| Absent | 1363 | 114 | 8.4 | 143 | 10.5 | 22 | 1.6 | 2 | 0.1 | 208 | 15.3 |
| Present | 36 | 9 | 25.0 | 16 | 44.4 | 2 | 5.6 | – | – | 18 | 50.0 |
| p value | p < 0.01 | p < 0.01 | p > 0.05 | – | p < 0.01 | ||||||
| Restricted tongue | |||||||||||
| Absent | 1398 | 123 | 8.8 | 159 | 11.4 | 24 | 1.7 | 2 | 0.1 | 226 | 16.2 |
| Present | 1 | – | – | – | – | – | – | – | – | – | – |
| p value | – | – | – | – | – | ||||||
| Total | 1399 | 123 | 8.8 | 159 | 11.4 | 24 | 1.7 | 2 | 0.1 | 226 | 16.2 |
Any addiction is defined as either use of smoking/tobacco chewing/alcohol/betel nut.
The distribution of various addictions among males and its comparison with age group, gender and symptoms are exhibited in Table 3. Majority of smokers (27.1%) belonged to age ≥55 years; tobacco chewers (29.2%) and alcohol users (45.8%) in the age group 25–34 years. The age distribution was significantly associated with the habit of alcohol use (p < 0.01). Nearly 25% of smokers and tobacco chewers were found with mouth ulcers however, 12.5% were alcohol users. More than 4% of alcohol users have been complaining of the symptom dysphagia. Roughly 10% in each smokers, tobacco chewers and alcohol users were screened positive for the symptom trismus. None of the symptoms were statistically associated with addictions in males except for tobacco chewing with trismus (p < 0.001).
Table 3.
Distribution and comparison of various addictions across demographic and various symptom variables among males.
| Variables | N | Distribution of addictions among males (n = 697) |
|||||
|---|---|---|---|---|---|---|---|
| Tobacco smoking (%) | p | Tobacco chewing (%) | p | Alcohol consumption (%) | p | ||
| Age group (years) | |||||||
| <25 | 103 | 12 (10.2) | 0.597 | 16 (10.4) | 0.062 | 2 (8.8) | 0.017 |
| 25–34 | 154 | 27 (22.9) | 45 (29.2) | 11 (45.8) | |||
| 35–44 | 140 | 25 (21.2) | 33 (21.4) | 7 (29.2) | |||
| 45–54 | 133 | 22 (18.6) | 30 (19.5) | 2 (8.3) | |||
| ≥55 | 167 | 32 (27.1) | 30 (19.5) | 2 (8.3) | |||
| Mouth ulcers | |||||||
| Absent | 536 | 89 (75.4) | 0.676 | 116 (75.3) | 0.599 | 21 (87.5) | 0.210 |
| Present | 161 | 29 (24.6) | 38 (24.7) | 3 (12.5) | |||
| Dysphasia | |||||||
| Absent | 688 | 116 (98.3) | 0.67 | 151 (98.1) | 0.413 | 23 (95.8) | 0.204 |
| Present | 9 | 2 (1.7) | 3 (1.9) | 1 (4.2) | |||
| Nose bleeding | |||||||
| Absent | 692 | 118 (100) | 0.311 | 154 (100) | 0.232 | 24 (100) | 0.672 |
| Present | 5 | 0 | 0 | 0 | |||
| Ear discharge | |||||||
| Absent | 686 | 116 (98.3) | 0.911 | 153 (99.4) | 0.295 | 24 (100) | 0.528 |
| Present | 11 | 2 (1.7) | 1 (0.6) | 0 | |||
| Neck swelling | |||||||
| Absent | 690 | 117 (99.2) | 0.851 | 154 (100) | 0.157 | 24 (100) | 0.616 |
| Present | 7 | 1 (0.8) | 0 | 0 | |||
| Hoarseness of voice | |||||||
| Absent | 678 | 114 (96.6) | 0.627 | 153 (99.4) | 0.073 | 24 (100) | 0.404 |
| Present | 19 | 4 (3.4) | 1 (0.6) | 0 | |||
| Trismus | |||||||
| Absent | 664 | 109 (92.4) | 0.105 | 138 (89.6) | 0.000 | 22 (91.7) | 0.398 |
| Present | 33 | 9 (7.6) | 16 (10.4) | 2 (8.3) | |||
| Total | 118 (16.9) | 154 (22.1) | 24 (3.4) | ||||
The multivariate logistic regression was used to predict the risk of different symptoms in the presence of age, sex and various addiction habits in Table 4. Male gender population (MRR = 35.7; 95% CI: 15.5–81.9) were significantly associated with the risk of mouth ulcers. Risk of trismus was statistically related with male gender (MRR = 7.7; 95% CI: 2.2–26.6) and tobacco chewers (MRR = 3.5; 95% CI: 1.6–7.5) with respect to their reference category.
Table 4.
Factors associated with symptoms using multivariate logistic regression analysis with 95% confidence interval.
| Variables | Mouth ulcers |
Dysphasia |
Trismus |
|||
|---|---|---|---|---|---|---|
| MRRa | 95% CI | MRRa | 95% CI | MRRa | 95% CI | |
| Age group (years) | ||||||
| <25 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| 25–34 | 1.5 | 0.8–2.7 | 1.7 | 0.2–16.4 | 0.9 | 0.3–2.7 |
| 35–44 | 1.7 | 0.9–3.3 | 1.2 | 0.1–13.1 | 1.2 | 0.4–3.8 |
| 45–54 | 1.1 | 0.6–1.9 | 2.3 | 0.3–21.2 | 0.9 | 0.3–3.0 |
| ≥55 | 0.9 | 0.5–1.8 | 0.5 | 0.1–8.3 | 0.4 | 0.1–1.5 |
| Gender | ||||||
| Male | 35.7f | 15.5–81.9 | 3.9 | 0.8–19.8 | 7.7f | 2.2–26.6 |
| Female | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Smoking | ||||||
| Present | 1.2 | 0.7–1.9 | 1.1 | 0.2–5.8 | 1.3 | 0.5–3.0 |
| Absent | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Tobacco chewing | ||||||
| Present | 1.1 | 0.7–1.8 | 1.4 | 0.3–6.9 | 3.5f | 1.6–7.5 |
| Absent | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
| Alcohol | ||||||
| Present | 0.3 | 0.1–1.2 | 2.9 | 0.2–33.9 | 0.7 | 0.1–3.5 |
| Absent | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | |||
CI, confidence interval; e, p < 0.05; f, p < 0.01; Ref., reference category.
Multivariate rate ratio.
4. Discussion
The oral cavity is an easily accessible site for screening by healthcare professionals or for self-examination. Visual screening has been shown to detect early oral neoplasia, if provided as a part of routine medical care by health workers.4 Mouth ulcers and trismus remained the common findings among the rural individuals.4 Early oral cancer cases have a better prognosis than those with advanced disease.15, 16, 17 However no definite evidence has been found so far to indicate that organized and systematic, population-based oral screening can reduce mortality from oral cancer. Rengaswamy et al. reported that due to effects of lead time and length bias, the observational data indicating detection of early stage cancers and the improved survival of early oral cancer cases are not sufficient evidence to recommend organized screening.12
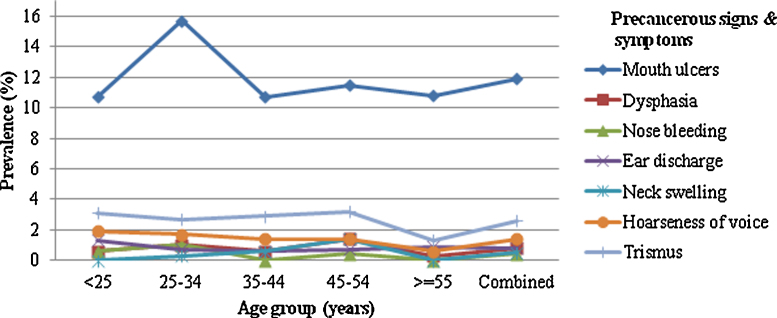
Overall, the primary symptoms were mouth ulcers (11.9%) followed by trismus (2.6%), hoarseness in voice (1.4%), dysphagia and ear discharge at 0.8% respectively. Symptoms among the smokers were trismus (25%), hoarseness of voice (21.1%), dysphagia (18.2%), ear discharge (18.2%), and mouth ulcers (17.4%). Symptoms among the tobacco chewers were trismus (44.4%), dysphagia (27.3%) and mouth ulcers (22.8%). The main symptom among alcohol users was found to be dysphagia (9.1%). However, a very small number of patients were found to have neck swelling (n = 7), nose bleeding (n = 5) and restricted tongue movement (n = 1) and no further statistical analysis has been designed for these symptoms as variables. The proportion of oral mucosal lesions in our study was higher (11.9%) in comparison to the previous studies from Vidisha4 (8.4%) and Chennai21 (4.1%). Ear discharge (1.3%) and hoarseness of voice (1.9%) were more prevalent in the lower age group (<25 years), mouth ulcers (15.7%), and nose bleeding (1%) were in age 25–34 years, trismus (3.2%), dysphagia (1.4%) and neck swelling (1.4%) were in the higher age group (45–54 years) (Fig. 1).
Fig. 1.
Prevalence of symptoms in different age groups.
Overall, the distribution of smoking, tobacco and alcohol was almost similar across the age groups and it was not statistically significant (p > 0.05). Due to the social and cultural practices, the prevalence of addictions was found more in male (217/697) compared to female (9/702). So, the prevalence of smoking (16.9%) and tobacco chewing (22.1%) was higher among males and is statistically associated (p < 0.001), when compared to the addiction pattern in females. It was found that the people were unaware of the fact that tobacco (smoking or chewing) use is the most important risk factor of cancer. These findings are in agreement with other published studies by Saraswathi et al.21 and Gupta et al.22
Prevalence of mouth ulcers among males was found to be 24.6%, who were addicted to smoking and 24.7% for who were addicted to tobacco chewing alone. However, higher prevalence rate of 32.8% was reported for those, who are addicted with both smoking and tobacco chewing. The prevalence of mouth ulcers among males was reported to be 12.5%, in subjects, who used to consume alcohol alone. However, 13.6% males were addicted with both tobacco chewing and alcohol. 6.3% of males were addicted with both smoking and alcohol consumptions, which might be due to the lesser numbers in the combined users of alcohol and smoking.
Occurrence of trismus among males was reported to be 7.6% with smoking, with tobacco chewers (10.4%) and with alcohol (8.3%) individually. However, it was observed having similar results with both smoking and tobacco (12.1%), with both smoking and alcohol (11.9%) and 2% in combined smokers and tobacco chewers; 4.2% of alcohol users were observed having dysphagia. Moreover, the prevalence of dysphagia remained low as the frequency of combined addictions was very small to conclude. Similarly, the prevalence of hoarseness of voice was not reported due to very limited number of data points for single as well as combined analysis.
The multivariable logistic regression analyses explored the causal relationship between different symptoms and demographic (age, sex), addiction (tobacco use and alcohol consumption) variables. Risk of developing mouth ulcers among males is more than 30-fold (MRR = 35.7) and trismus is nearly 8-fold (MRR = 7.7) with compared to females. None of the addictions were statistically related alone with the symptoms except for tobacco chewing with trismus (MRR = 3.5). Further, the age and sex adjusted multivariate analysis estimated that the combined use of smoking and tobacco have more than 2-fold (MRR = 2.6) risk of developing mouth ulcers and 3-fold (MRR = 3.1) likelihood of developing trismus with respect to non-users (Table 5). Saraswathi et al. has also reported that smoking and tobacco chewers are independent risk factors for head and neck cancer.21
Table 5.
Effect of different combination of addiction habits on prevalence of symptoms among males.
| Combination of addiction habits among males | N | Mouth ulcers (%) | MRR (95% CI) | Dysphasia (%) | MRR (95% CI) | Trismus (%) | MRR (95% CI) |
|---|---|---|---|---|---|---|---|
| Smoking and tobacco | |||||||
| Absent | 639 | 142 (22.2) | 1 (Ref.) | 8 (1.3) | 1 (Ref.) | 26 (4.1) | 1 (Ref.) |
| Present | 58 | 19 (32.8) | 2.6f (1.4–5.0) | 1 (1.7) | 2.2 (0.3–18.2) | 7 (12.1) | 3.2f (1.2–8.7) |
| Smoking and alcohol | |||||||
| Absent | 681 | 160 (23.5) | 1 (Ref.) | 9 (1.3) | – | 31 (4.6) | 1 (Ref.) |
| Present | 16 | 1 (6.3) | 0.3 (0.1–1.7) | – | – | 2 (12.5) | 2.9 (0.6–13.7) |
| Tobacco and alcohol | |||||||
| Absent | 675 | 158 (23.4) | 1 (Ref.) | 8 (1.2) | 1 (Ref.) | 31 (4.6) | 1 (Ref.) |
| Present | 22 | 3 (13.6) | 1.8 (0.4–9.7) | 1 (4.5) | 1.7 (0.8–2.8) | 2 (9.1) | 2.1 (0.5–9.3) |
Adjusted for age and sex; MRR, multivariate rate ratio; CI, confidence interval; f, p < 0.01; Ref., reference category.
The present analysis showed a strong association between tobacco use (smoked or smokeless) and mouth ulcers and trismus in male gender. However, there was a weak association of mouth ulcers, dysphagia and trismus with age. An association of symptoms with smoking, tobacco chewing and other addictions has also previously been reported by Rengaswamy et al.12 Majority of male smokers (27.1%) belonged to age ≥55 years, whereas the tobacco chewers (29.2%) and alcohol users (45.8%) in the age group of 25–34 years. The alcohol consumption is more prevalent in younger age (<35 years) with compared to higher age (p < 0.05).21
Among the females, the prevalence of addiction was very less, which may be due to socio cultural reasons, hence the chances of developing the symptoms of head neck malignancy were also found to be very less. This gives indirect evidence that the following symptoms (mouth ulcers, trismus, dysphagia, etc.) are related to the addiction habits in males and minimally due to other associated causes like malnutrition, vitamin deficiency, etc.21
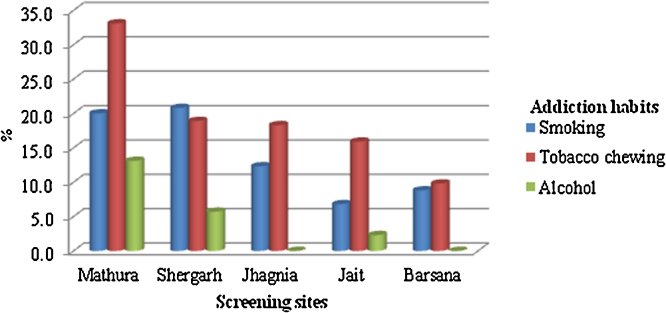
The prevalence of tobacco use (smoked and smokeless) and alcohol consumptions was highest at Mathura site followed by Shergarh respectively (Fig. 2). Tobacco chewing is predominantly used by the screened individuals at all locations. Consumption of alcohol was absent among the individuals, who turned up for screening except from Mathura, Shergarh and Jait locations. The consumption of tobacco use (smoked or smokeless) is subject to geographical variations and showed marked differences between rural (among males, 50%; among females, 17%) and urban (among males, 30%; among females, 3%) societies.22, 23, 24 It indicates that there was low awareness of ill effects of tobacco addiction and marked rise in the tobacco use (smoked and smokeless) and associated products in rural scenario.25, 26
Fig. 2.
Screening sites with higher prevalence of addictions.
Though the study provides useful information related to oral ulcers and its association with addictions, however it has some limitations also. Findings are based on the screened individuals, who came for the general health screening at camps. Therefore it may not be the representation of general population. This analysis is based on cross-sectional data and some categories of explanatory variables did not have an adequate number of observations to estimate the odds ratios. It was not possible to report the prevalence of carcinoma in the present study due to lack of histopathological confirmation. Further studies with larger cohort and histopathology confirmation are being planned. Of the clinically significant lesions, those were diagnosed in our study, pre-cancerous lesions like leucoplakia versus autoimmune etiologies like lichen planus could not be differentiated. Since, the occurrence of symptoms is a multi-factorial phenomenon. Therefore the recorded symptoms may be due to some other unrelated reasons too, namely SLE, vitamin deficiencies, malnutrition, etc.
5. Conclusion and future directions
In light of the existing evidences, this study highlights that we have very high propensity oral ulcers in the screened population and the addiction of smokeless tobacco. Mouth ulcers and trismus were common symptoms and tobacco use (smoking or chewing) was found to be the leading addiction in the rural population of western Uttar Pradesh and eastern Rajasthan, India. Single or combined use of addictions among males was associated with the risk of developing precancerous signs and symptoms. Along with the community, the health workers were also sensitized and trained for oral and self examination and collection of cancer related data. Results of the present study indicates that organized visual screening of oral cavity is a worthwhile initiative in early detection and control of oral cancer by diagnosing early mucosal changes in oral cavity and predictors of precancerous lesions, in addition to primary prevention efforts to reduce tobacco (smoking or chewing) and alcohol abuse.
Further, this screening programme will be extended at larger level subsequently in the next phases with histopathological testing facility that includes programme sensitivity (screen-detected oral cancer as a proportion of the total oral cancer cases diagnosed in the intervention group) and positive predictive value (proportion of positive screening results with a reference diagnosis of pre-cancer or oral cancer).
Conflicts of interest
The authors have none to declare.
Acknowledgements
Authors are thankful to Ms Niira Radia, Dr. R.K. Mani, for their support and valuable suggestions. We also would like to acknowledge Mr Akash Radia, Col Suresh Bhatt, Ms Mamata Dasgupta and the outreach team, who collected the data.
Contributor Information
Santanu Chaudhuri, Email: chaudhurisantanu@hotmail.com.
Somnath Dey, Email: drdeysomnath@yahoo.co.in.
Ram Chandra Bajpai, Email: rambajpai@hotmail.com.
References
- 1.Silverman S. Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132(suppl) doi: 10.14219/jada.archive.2001.0382. 7S–11S. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni M.R. Head and neck cancer burden in India. Int J Head Neck Surg. 2013;4(1):25–29. [Google Scholar]
- 3.Gupta P.C., Sinor P.N., Bhonsle R.B., Pawar V.S., Mehta H.C. Oral submucous fibrosis in India: a new epidemic? Natl Med J India. 1998;11(3):113–116. [PubMed] [Google Scholar]
- 4.Mehrotra R., Thomas S., Nair P. Prevalence of oral soft tissue lesions in Vidisha. BMC Res Notes. 2010;3:23. doi: 10.1186/1756-0500-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warnakulasuriya K.A.A.S., Ekanayake A.N.I., Sivayoham S. Utilisation of primary care workers for early detection of oral cancer and precancer cases in Sri Lanka. Bull World Health Organ. 1984;62(2):243–250. [PMC free article] [PubMed] [Google Scholar]
- 6.Warnakulasuriya K.A.A.S., Nanayakkara B.G. Reproducibility of an oral cancer and precancer detection program using a primary health care model in Sri Lanka. Cancer Detect Prev. 1991;15(5):331–334. [PubMed] [Google Scholar]
- 7.Mehta F.S., Gupta P.C., Bhonsle R.B., Murti P.R., Daftary D.K., Pindborg J.J. Detection of oral cancer using basic health workers in an area of high oral cancer incidence in India. Cancer Detect Prev. 1986;9(3–4):219–225. [PubMed] [Google Scholar]
- 8.Mathew B., Sankaranarayanan R., Sunilkumar K.B., Binu K., Pisani P., Krishnan Nair M. Reproducibility and validity of oral visual inspection by trained health workers in the detection of oral precancer and cancer. Br J Cancer. 1997;76(3):390–394. doi: 10.1038/bjc.1997.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew B., Wesley R., Dutt S.C., Amma S., Sreekumar C. Cancer screening by local volunteers. World Health Forum. 1996;17(4):377–378. [PubMed] [Google Scholar]
- 10.Sankaranarayanan R. Healthcare auxiliaries in the detection and prevention of oral cancer. Oral Oncol. 1997;33(3):149–154. doi: 10.1016/s0964-1955(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 11.Lawal F.B. Global self-rating of oral health as summary tool for oral health evaluation in low-resource settings. J Int Soc Prev Community Dent. 2015;5(suppl 1):S1–S6. doi: 10.4103/2231-0762.156516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rengaswamy S., Ramadas K., Thomas G. Effect of screening on oral cancer mortality in Kerala, India: a cluster-randomised controlled trial. Lancet. 2005;365(9475):1927–1933. doi: 10.1016/S0140-6736(05)66658-5. [DOI] [PubMed] [Google Scholar]
- 13.Mathew B. Regional Cancer Centre; Trivandrum: 1988. A Guide for Health Workers on Early Detection of Oral Cancer. [Google Scholar]
- 14.Mehta F.S., Hamner J.E., III. Jaypee Brothers Medical Publishers; New Delhi: 1993. Tobacco Related Oral Mucosal Lesions and Conditions in India. [Google Scholar]
- 15.Ries L.A.G., Kosary C.L., Hankey B.F., Miller B.A., Harras A., Edwards B.K. National Cancer Institute; Bethesda, MD: 1997. SEER Cancer Statistics, Review: 1973–1994. [Google Scholar]
- 16.Sankaranarayanan R., Black R.J., Parkin D.M. IARC; Lyon: 1998. Cancer Survival in Developing Countries. [PubMed] [Google Scholar]
- 17.Yeole B., Sankaranarayanan R., Sunny L., Swaminathan R., Parkin D.M. Survival from head and neck cancer in Mumbai (Bombay), India. Cancer. 2000;89(2):437–444. doi: 10.1002/1097-0142(20000715)89:2<437::aid-cncr32>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.de Martins A.M.E., Souza B.L.J.G.S., Haikal D.S., Paula A.M.B., de, Ferreira E.F.E., Pordeus I.A. Prevalence of oral cancer self-examination among elderly people treated under Brazil's Unified Health System: household health survey. Ciênc Saúde Coletiva. 2015;20(4):1085–1098. doi: 10.1590/1413-81232015204.00542014. [DOI] [PubMed] [Google Scholar]
- 19.Siegel S., Castellan J. 2nd ed. McGraw-Hill; New York, NY: 1988. Nonparametric Statistics for the Behavioral Sciences. [Google Scholar]
- 20.Hosmer D.W., Lemeshow S. 3rd ed. John Wiley & Sons; New York, NY: 2000. Applied Logistic Regression. [Google Scholar]
- 21.Saraswathi T.R., Ranganathan K., Shanmugam S., Sowmya R.N., Narasimhan P.D., Gunaseelan R. Prevalence of oral lesions in relation to habits: cross-sectional study in South India. Indian J Dent Res. 2006;17(3):121–125. doi: 10.4103/0970-9290.29877. [DOI] [PubMed] [Google Scholar]
- 22.Gupta V., Yadav K., Anand K. Patterns of tobacco use across rural, urban, and urban-slum populations in a North Indian community. Indian J Community Med. 2010;35(2):235–251. doi: 10.4103/0970-0218.66877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torwane N.A., Hongal S., Goel P., Chandrashekar B., Saxena V. Assessment of oral mucosal lesions among eunuchs residing in Bhopal City, Madhya Pradesh, India: a cross-sectional study. Indian J Public Health. 2015;59(1):24–29. doi: 10.4103/0019-557X.152851. [DOI] [PubMed] [Google Scholar]
- 24.Chadda R., Sengupta S. Tobacco use by Indian adolescents. Tob Induc Dis. 2003;1(1):8. doi: 10.1186/1617-9625-1-2-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinha D., Gupta P., Ray C., Singh P. Prevalence of smokeless tobacco use among adults in WHO South-East Asia. Indian J Cancer. 2012;49(4):342–346. doi: 10.4103/0019-509X.107726. [DOI] [PubMed] [Google Scholar]
- 26.Sujatha D., Hebbar P.B., Pai A. Prevalence of correlation of oral lesions among tobacco smokers, tobacco chewers, areca nut and alcohol users. Asian Pac J Cancer Prev. 2012;13(4):1633–1637. doi: 10.7314/apjcp.2012.13.4.1633. [DOI] [PubMed] [Google Scholar]