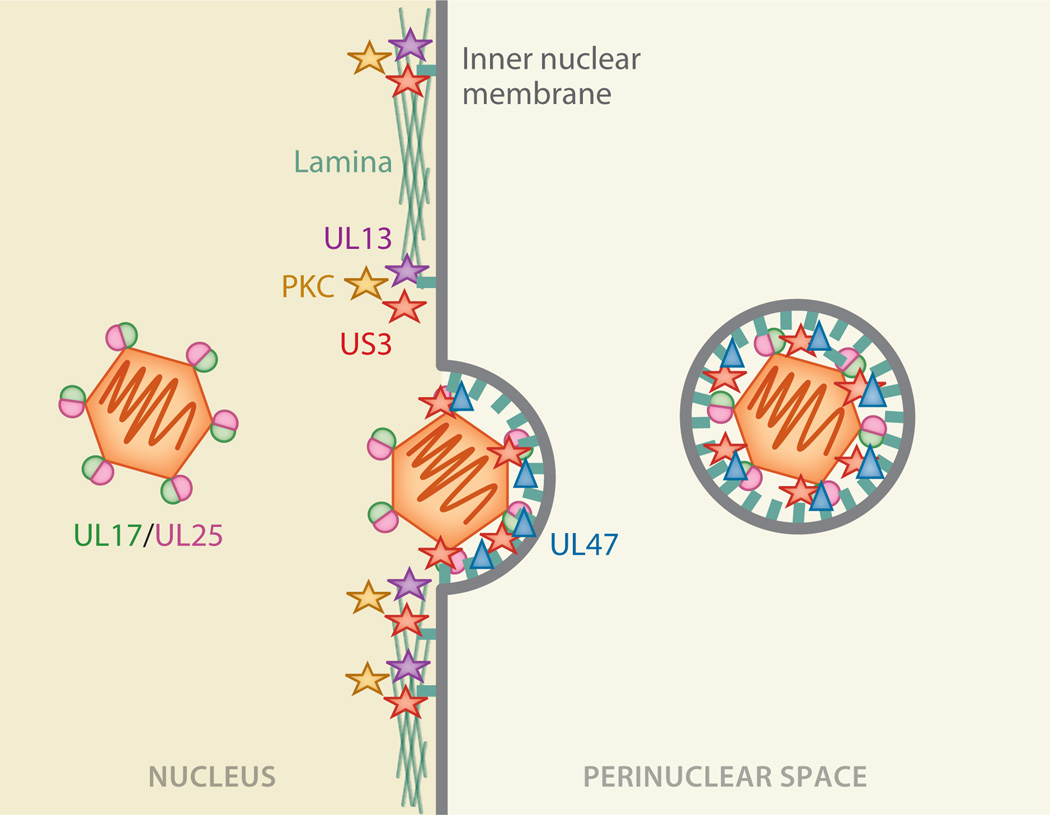
Figure 3.
Multiple proteins are involved in primary envelopment (nuclear budding). Although the nuclear egress complex can mediate vesicle budding by itself in vitro, this process appears subject to positive and negative regulation by a number of proteins during infection. Cellular and viral kinases (e.g., UL13, US3, and PKC) are recruited to the sites of primary envelopment for herpesviruses of all subfamilies. They phosphorylate proteins within the nuclear lamina as well as in the nuclear egress complex itself. This loosens the stiff lamina and allows capsids to be recruited to the inner nuclear membrane. Other viral proteins, such as UL47, have been implicated in regulating efficient nuclear egress, but their precise roles are still unclear. The nuclear egress complex is also responsible for capsid recruitment and may do so by binding the accessory capsid proteins UL17/UL25 or the major capsid protein VP5.