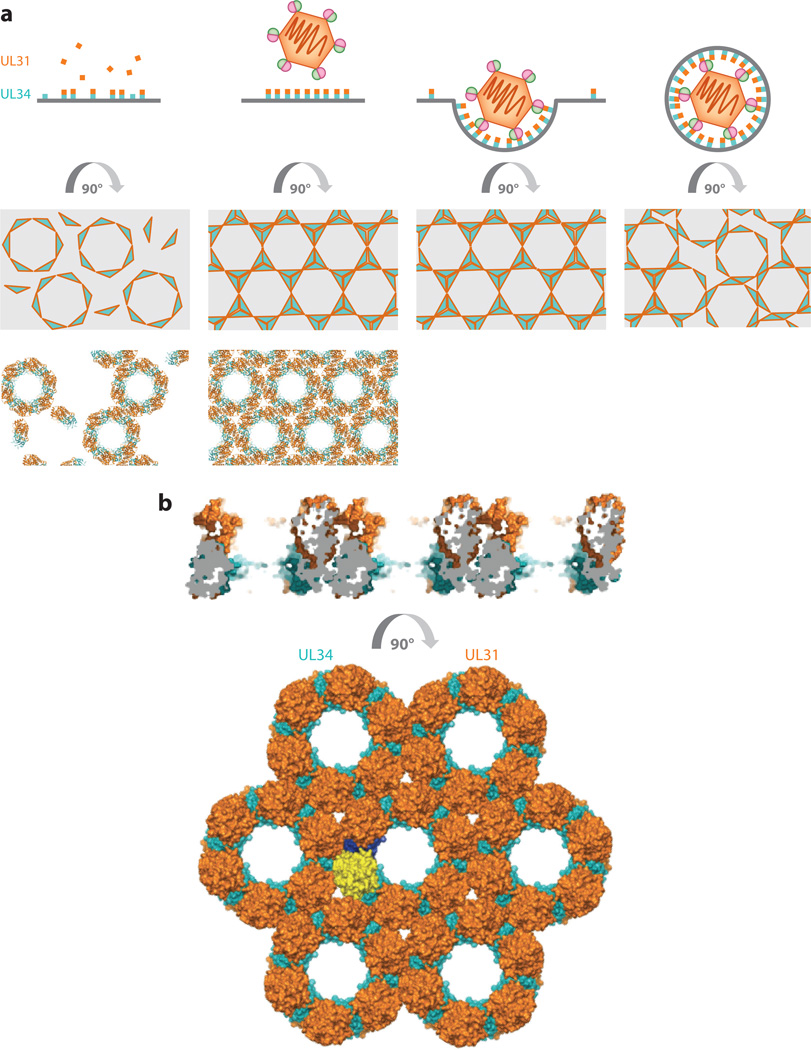
Figure 4.
Nuclear egress complex coat formation drives membrane deformation and budding. (a) UL34 (teal), anchored in the inner nuclear membrane, binds UL31 (orange) to form the nuclear egress complex heterodimer. The complex initially forms hexameric rings that eventually extend into a larger lattice when the capsid approaches. Conformational changes likely deform the membrane initially, but larger structural rearrangements are needed for sphere formation. This is possibly achieved by the introduction of errors within the hexagonal lattice. (b) The herpes simplex virus (HSV)-1 nuclear egress complex forms crystalline hexagonal lattices (PDB: 4ZXS), which resemble the hexagonal coats observed by cryo–electron tomography. The detailed analysis of the lattice allowed the identification of regions important for coat formation and membrane budding. Figure adapted with permission from Reference 38.