Figure 2.
Physical Proximity between Sister Chromatids Maintains SCIs Post-replication
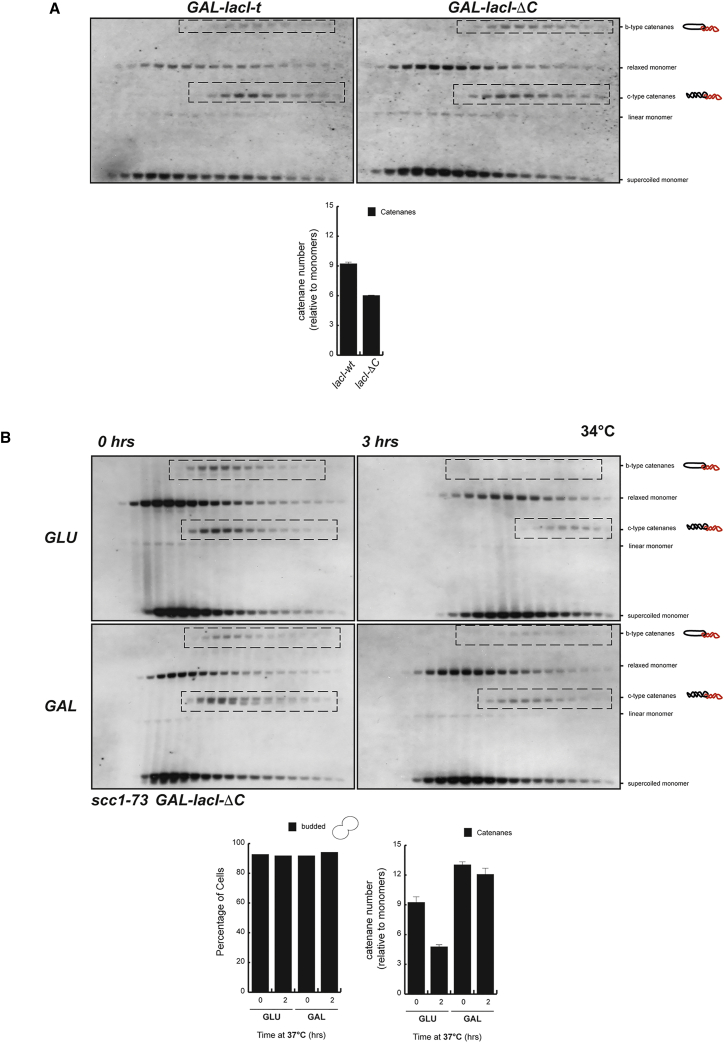
(A) Strains bearing a 10 kb circular minichromosome containing short lacO arrays and expressing a wild-type lacI (lacI-t; able to bind two operators simultaneously) or a lacI with a C-terminal deletion (lacI-ΔC; able to bind a single operator) under the galactose promoter were synchronized in G1 and released into the cell cycle in the presence of galactose and nocodazole at 30°C. Cell extracts were separated by differential sedimentation, heat denatured in 1% SDS, and analyzed by Southern blotting to reveal plasmid species. Plasmid species included monomeric forms (OCm, Lm, and CCCm) and dimeric forms, including different SCI species (Catc-type catenanes and Catb-type catenanes). SCIs are indicated in the blots (dashed boxes). SCIs (including c- and b-type catenanes) were quantified relative to monomeric species using ImageJ software (right-hand graph; showing the mean ± SD from three independent experiments). Cells expressing lacI-t exhibited a small increase in SCI levels.
(B) The scc1-73 strain bearing the 10 kb circular minichromosome containing the lacO array and the lacI-t construct under the galactose promoter was grown in raffinose media and synchronized in G1. The culture was divided in two. One half was released in galactose media (expressing lacI-t) while the other half was released in glucose media (repressing lacI-t). Both cultures were released from the G1 arrest in the presence of nocodazole at 25°C, and upon metaphase arrest, the cultures were shifted to 34°C to inactivate scc1-73. Samples were analyzed at the metaphase block at 25°C and 3 hr after temperature shift. Analysis was done as in (A). scc1-73 inactivation led to reduced SCI levels in the absence of lacI-t expression, but not in its presence. SCIs were quantified as in (A) (bottom right-hand graph; showing the mean ± SD from three independent experiments).