Figure 4.
Increased Levels of Cellular Top2 Negatively Affect Sister Chromatid Separation
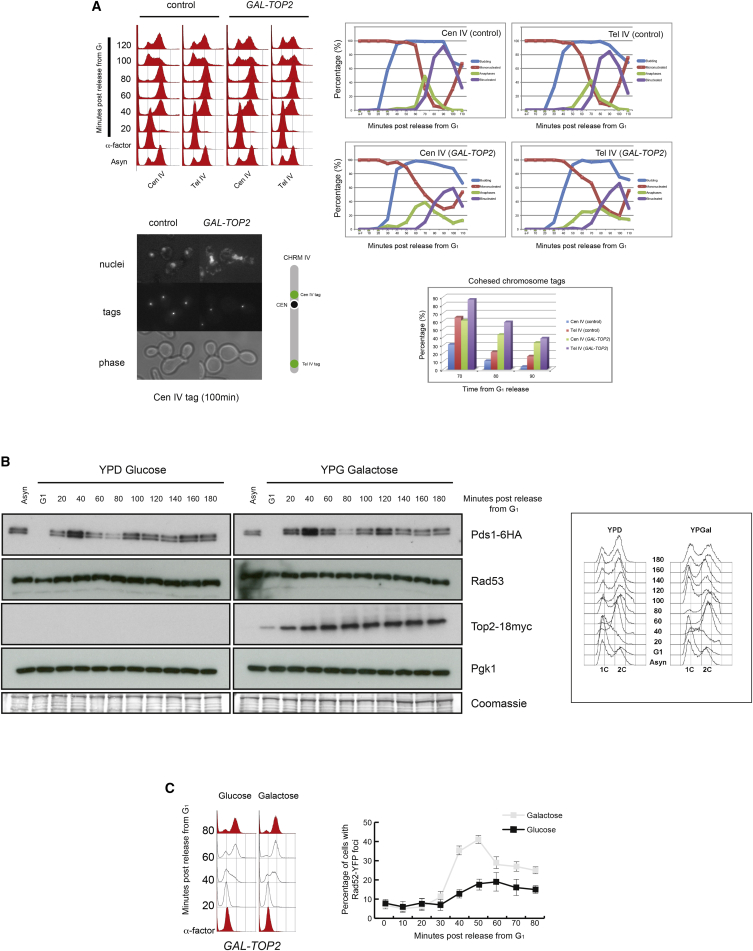
(A) Wild-type and GAL-TOP2 strains, carrying chromosomal tag (tetO) insertions in the vicinity of the centromere (Cen IV) or right telomere (Tel IV) of chromosome IV, were synchronized in G1 in media containing raffinose and released into the cell cycle in the presence of galactose at 30°C. Cytological analysis involved imaging 200 cells per time point in each replicate experiment. Percentage of mononucleated cells, binucleated cells, and anaphases (stretched DNA mass across bud-neck), together with the budding index, is shown for each strain. Sister chromatid separation dynamics in the strains was measured by scoring cohesed chromosomal tags at time points between 70 and 90 min (bottom graph). FACS profile for each strain is shown. Representative micrographs for wild-type and GAL-TOP2 strains carrying centromeric tags are shown. Diagrammatic representation of the position of the chromosomal tags within chromosome IV is indicated.
(B) A strain carrying GAL-TOP2-18myc Pds1-6HA was synchronized in G1 in media containing raffinose and released into the cell cycle in the presence of glucose or galactose at 30°C. Samples were taken for analysis at the times indicated. Top2 induction, Pds1 degradation, and Rad53 phophorylation during the time courses were followed by western blot. FACS profile is shown. No changes in Rad53 phosphorylation or Pds1 degradation timing were observed in cells overexpressing TOP2.
(C) A GAL-TOP2 strain bearing RAD52-YFP constructs was synchronized in G1 in media containing raffinose and released into the cell cycle in the presence of glucose or galactose at 30°C. Samples were taken for analysis every 10 min as the cultures proceeded through S phase and analyzed microscopically for Rad52 foci formation and by FACS. Cytological analysis involved imaging 200 cells per time point in each replicate experiment.