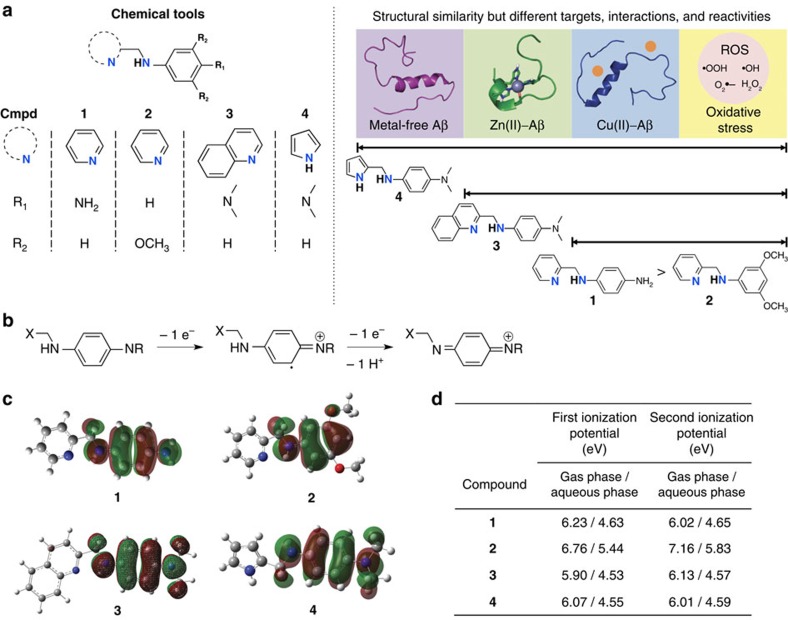
Figure 1. Targets associated with AD and ionization potentials of chemical tools (1–4).
(a) Structures of 1–4 [1, N1-(pyridin-2-ylmethyl)benzene-1,4-diamine; 2, 3,5-dimethoxy-N-(pyridin-2-ylmethyl)aniline; 3, N1,N1-dimethyl-N4-(quinolin-2-ylmethyl)benzene-1,4-diamine; 4, N1-((1H-pyrrol-2-yl)methyl)-N4,N4-dimethylbenzene-1,4-diamine] and their targets (metal-free Aβ, Cu(II)–Aβ, Zn(II)–Aβ and ROS). (b) Scheme of the oxidation of p-phenylenediamines. (c) Isosurface plot of SOMOs of cationic radicals of 1–4 with an isovalue of 0.02 au (red: O; blue: N; grey: C; white: H). (d) Calculated ionization potentials for 1–4 for the first and second processes depicted in b in both the gas and aqueous phases.