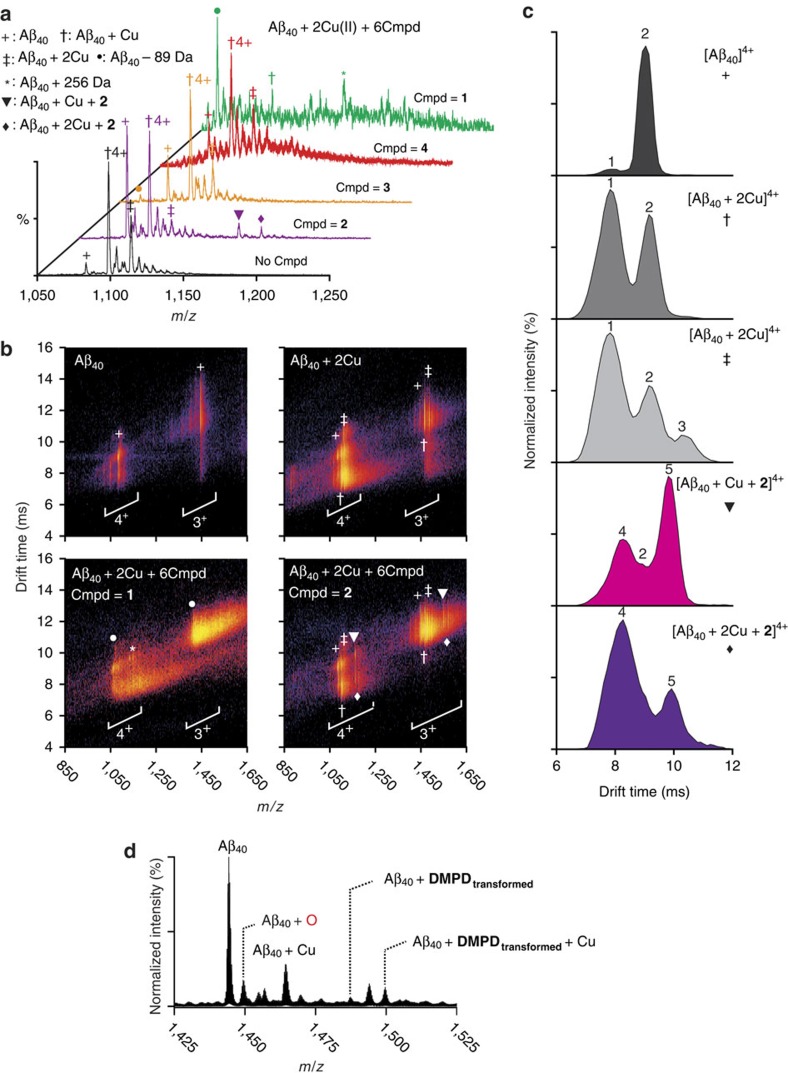
Figure 5. ESI–MS and IM–MS analyses of 1–4 in the presence or absence of Cu(II).
(a) Metal-dependent interactions of 1–4 with monomeric Aβ40. Both 1 and 3 promote the formation of Aβ mass loss product 89 Da lighter than the metal-free peptide (●), consistent with previously published data25. Incubations of Aβ with 1 were additionally shown to produce an adduct consistent with a mass gain of 256 Da compared with the intact, unmodified, peptide (*). 2 forms stable ternary complexes with Aβ40 and Cu(II), existing in two different stoichiometries that contain either one or two equivalents of the metal (▾ and ♦, respectively). Conditions for (a): [Aβ40]=18 μM; [copper(II) acetate]=40 μM; [1–4]=120 μM; 100 mM ammonium acetate (pH 7.5) with 1% v/v DMSO; 37 °C; 30 min incubation. (b) IM drift time versus m/z plots comparing the data acquired for 1 (bottom left) and 2 (bottom right) against Cu(II)-bound Aβ40 (top right) and Cu-free Aβ40 (top left). (c) Arrival time distribution data extracted from the plots which provide that these stable binding interactions support altered distributions upon binding to 2 when compared with the metal-free and Cu-bound Aβ40 complexes (collision cross section (CCS) data presented in Supplementary Table 4). (d) ESI–MS spectra of Aβ40 with 4 in the presence of Cu(II) under different conditions from (a). Aβ–DMPDtransformed complexes and oxidized Aβ40 were found when Cu(II) was present. The different positive charge states (with H+ and Na+) are used to best represent the complexes observed. Conditions for d (actual injected [Aβ]=10 μM): [Aβ40]=100 μM; [Cu(II)]=100 μM; [4]=500 μM; 100 mM ammonium acetate (pH 7.5) with 1% v/v DMSO; 37 °C; 1 h incubation.